| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 4, Number 5-6, December 2014, pages 121-135
Associations Between Liver Function, Bone Turnover Biomarkers and Adipokines in Older Patients With Hip Fracture
Leon Fishera, d, Alexander Fisherb, c
aDepartment of Gastroenterology, The Canberra Hospital, Canberra, ACT, Australia
bDepartment of Geriatric Medicine, The Canberra Hospital, Canberra, ACT, Australia
cAustralian National University Medical School, Canberra, ACT, Australia
dCorresponding Author: Leon Fisher, The Canberra Hospital, PO Box 11, Woden, ACT 2606, Australia
Manuscript accepted for publication December 01, 2014
Short title: Liver Function, Bone Turnover and Adipokines
doi: http://dx.doi.org/10.14740/jem250w
| Abstract | ▴Top |
Background: To examine the associations of serum liver markers with parameters of mineral and bone metabolism and their relationship with leptin, adiponectin and resistin in patients with hip fracture (HF).
Methods: In 294 older patients (mean age 82.2 ± 7.9 years, 72.1% women) with osteoporotic HF, we measured serum levels of alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), bilirubin, albumin, adiponectin, leptin, resistin, 25(OH) vitamin D (25(OH)D), intact parathyroid hormone (PTH), calcium, phosphate, magnesium, osteocalcin (OC), bone-specific alkaline phosphatase (BAP), urinary concentrations of deoxypyridinoline (DPD/Cr) and cross-linked N-telopeptide of type 1 collagen (NTx/Cr) (both corrected for urinary creatinine concentrations), as well as routine blood parameters.
Results: In the total cohort, in fully adjusted multivariate linear regression analyses, lower OC was an independent predictor of higher GGT, ALT and bilirubin, whereas higher BAP was positively associated with GGT and ALP; NTx/Cr, hemoglobin (both inversely), adiponectin, coronary artery disease (CAD) and alcohol overuse (all three positively) were also independently associated with GGT activity. However, in malnourished women, OC was not an independent predictor of GGT or ALT and NTx/Cr predicted ALP activity. OC was independently predicted by GGT, ALT (both negatively), ALP, leptin and age (all three positively), BAP by GGT and ALP, OC/BAP ratio by GGT (inversely) and leptin (positively) and both elevated NTx/Cr and DPD/Cr by higher ALP and lower leptin levels. The GGT > 20 U/L indicated increased prevalence of low OC levels (two-fold) and low OC/BAP ratio (2.6-fold) with a positive predictive value above 75%.
Conclusions: In older HF patients, bidirectional links exist between liver function (within normal range in the vast majority) and parameters of bone metabolism. Adiponectin is an independent predictor of GGT, whereas leptin is a determinant of OC and bone resorption; these relationships are modulated by nutritional status. GGT > 20 U/L may be used as a marker of impaired bone metabolism.
Keywords: Liver function; Adipokines; Bone turnover; Osteocalcin
| Introduction | ▴Top |
The importance of liver-bone interactions under both normal and disease conditions is supported by a growing number of evidence. 1) Liver plays a fundamental role in metabolism of vitamins D and K, parathyroid hormone (PTH) and minerals, essential regulators of bone homeostasis [1-5]. 2) Osteopenia/osteoporosis (hepatic osteodystrophy) is present in 20-50% of patients with chronic liver disease [6-10]. 3) Osteocalcin (OC), an osteoblast-derived hormone, is recognized as a critical determinant of energy and glucose homeostasis [11-16]. 4) Dysregulation and dysfunction of adipokines, adipose tissue-derived hormones, in particular, adiponectin, leptin and resistin, which have receptors expressed in both hepatocytes and bone cells and control a vast diversity of physiological functions, are involved in initiation and progression of many diseases including liver and bone (osteoporosis) disorders [17-23].
However, despite the intense research carried out in recent years, the relationship between liver and bone and the underlying mechanisms, including the role of adipokines, are still poorly characterized. To date, neither clinical, nor the animal models provide a uniform explanation of the liver-bone link(s). The majority of research on adipokine-liver-bone interactions has focused primarily on obesity-related metabolic syndrome, diabetes mellitus (DM) and non-alcoholic fatty liver disease (NAFLD). There has been limited evaluation (with considerable controversy in the reports) of liver function, bone metabolism and adipokines in patients with hip fracture (HF), the most devastating consequence of osteoporosis. There are no data concerning the association of serum hepatic biomarkers with indices of mineral and bone metabolism and the mechanisms involved, nor are there any data showing whether liver function indicators predict clinically important markers of bone turnover in HF patients. Thus, the aims of this study were to evaluate 1) the cross-sectional associations of serum liver markers with parameters of mineral and bone metabolism, and 2) the relationship between serum levels of three adipokines (leptin, adiponectin and resistin) and both liver and mineral-bone indicators in older patients with HF.
| Methods | ▴Top |
Study cohort
This prospective observational study included 294 consecutive older (≥ 60 years of age) patients (212 women and 82 men) with low-trauma osteoporotic HF admitted to the Canberra Hospital who did not have the following exclusion criteria: age < 60 years, high trauma, femur shaft fracture, pathological HF due to primary or metastatic bone cancer, multiple myeloma, Paget disease, or primary hyperparathyroidism. Data on sociodemographic, clinical and laboratory characteristics were collected as previously reported [24].
The study was conducted in compliance with the Declaration of Helsinki (as revised in 2008).
Informed consent was obtained from all patients or their carers. The study has approval of the local Health Human Research Ethical Committee.
Laboratory analyses
In each patient venous blood and second morning urine samples were collected between 07:00 and 11:00 after at least 12-h overnight fast, usually within 24 h after arrival at the emergency department. After centrifugation of blood, one serum sample as well as the urine sample was frozen and stored at -70 °C for later analyses of bone turnover markers and adipokines.
For all patients, the following parameters were measured: complete blood count, liver function tests, urea, creatinine and electrolytes, fasting blood glucose (and HbA1C in diabetic patients), thyroid function tests (TSH, T4), 25(OH) vitamin D (25(OH)D), intact PTH, total calcium, phosphate, magnesium, markers of bone turnover, and three adipokines (adiponectin, leptin and resistin). All routine hematological and biochemical assessments were performed by standardized methods on autoanalyzers at the day of collection. Glomerular filtration rate (eGFR) was calculated using a standardized serum creatinine-based formula normalized to a body surface area of 1.73 m2 [25]. Chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73 m2 (CKD stage ≥ 3).
Assessment of liver parameters
The following biochemical indicators of liver function were measured as the blood samples were collected: alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), bilirubin and albumin. These markers were evaluated by using commercially available standard enzymatic reagents and diagnostic kits by spectrophotometry on the biochemical autoanalyzer Abbott Architect CI16200 (Abbott Laboratories, IL, USA). ALT, GGT and ALP were measured with enzymatic methods, total bilirubin was analyzed using diazonium salt, albumin was measured using bromcresol green, and total protein was tested by a Biuret method. The mean inter-assay and intra-assay coefficients of variations (CVs) for these tests were within 1.1-6.6%. For liver enzymes two times the upper normal limit (UNL), cut-off levels were used to define abnormal tests.
Markers related to mineral and bone metabolism
These included serum concentrations of 25(OH)D, intact PTH, total calcium, phosphate, magnesium, and markers of bone turnover - OC, and bone-specific alkaline phosphatase (BAP) as markers of bone formation and urinary concentrations of deoxypyridinoline (DPD/Cr), and cross-linked N-telopeptide of type 1 collagen (NTx/Cr) as markers of bone resorption (both corrected for urinary creatinine concentrations in the same sample). Serum calcium concentrations were corrected for serum albumin. Serum 25(OH)D was measured by radioimmunoassay kit (Dia Sorin, Stillwater, MN, USA) and intact PTH by two-site chemiluminescent enzyme-linked immunoassay on DPC Immulite 2000 (Diagnostic Products Corp., Los Angeles, CA, USA). Serum OC was determined by electrochemiluminescent immunoassay (Elecsys 1010, Roche Diagnostics, Ltd Corp., IN, USA), serum BAP by Metra BAP ELISA (Quidel Corp., San Diego, CA, USA), urinary NTx by enzyme-linked immunosorbent assay (ELISA) (Wampole Labs, Princeton, NJ, USA), and urinary DPD by two-site chemiluminescent enzyme-labeled immunoassay (DPC Immulite 2000, Diagnostic Products, Los Angeles, CA, USA). All samples were analyzed with commercially available kits of the same lot number according to the manufacturer’s protocol and blind to any clinical information. In these methods both the intra- and inter-assay CVs ranged from 2.1% to 12.7%. Insufficiency of vitamin D was defined as 25(OH)D < 50 nmol/L and secondary hyperparathyroidism (SHPT) was defined as elevated serum PTH (> 6.8 pmol/L, the upper limit of the laboratory reference range). For levels of bone turnover markers, we used the standard laboratory reference ranges and data provided by the manufacturer.
Measurements of adipokines
Serum levels of leptin were determined by ELISA method (Diagnostic System Laboratories, Webster, TX, USA), and total adiponectin and resistin by human ELISA kits (B-Bridge International, Mountain View, CA, USA). All assays were performed according to the manufactures’ instructions with kits of the same lot number. Intra- and inter-assay CVs were less than 7% for these three tests. Malnutrition was defined as serum leptin concentration < 4 ng/mL in males and < 6.5 ng/mL in females [26].
Statistical analyses
Data are presented as mean values ± standard deviations (SDs) for continuous variables or percentages for categorical variables. Comparisons between groups were performed using analysis of variance and Student’s t-test (for continuous normally distributed variables) and χ2 test (for categorical variables). The relations between variables were also analyzed by Pearson’s correlation coefficient with log-transformed data (to achieve normal distribution); Bonferroni and Sidak adjustments for multiplicity have been performed. Univariate and multivariate linear regression analyses were performed to determine the associations between liver markers and different parameters related to mineral and bone metabolism; all potential confounding variables (demographic, biochemical and clinical) with statistical significance ≤ 0.10 on univariate analyses were included in multivariate analysis. The significance of multicollinearity phenomena in regression analyses was evaluated by the variance inflation factor. Two-sided P < 0.05 values were considered statistically significant. All statistical calculations were carried out using the Stata software version 10 (StataCorp, College Station, TX, USA).
| Results | ▴Top |
Clinical characteristics of the study patients
The clinical features and laboratory characteristics in the patients are summarized in Table 1. The prevalence of abnormal liver enzyme activities defined as two times over their respective ULNs was low or moderate: for ALT (> 80 U/L) in five (1.7%) patients, for GGT (> 128 U/L) in 23 (7.8%), and for ALP (> 120 U/L) in 26 (8.8%) subjects. Elevated bilirubin (> ULN, 20 µmol/L) demonstrated 29 (9.9%) patients and low albumin levels (< 33 g/L) were found in 64 (21.8%) subjects. There were no gender differences in mean values of liver markers, OC, BAP, calcium (corrected for albumin), resistin, TSH and hemoglobin. Women, compared to men, had significantly lower mean serum 25(OH)D (35.3 ± 17.6 vs. 42.4 ± 18.2 nmol/L, P = 0.003) and eGFR (62.7 ± 22.2 vs. 71.2 ± 26.4 mL/min/1.73 m2, P = 0.006) levels, but higher concentrations of PTH (7.4 ± 6.1 vs. 5.5 ± 3.5 pmol/L, P = 0.009), NTx/Cr (178.0 ± 90.5 vs. 112.3 ± 78.0 nmol/µmol, P = 0.005), DPD/Cr (13.3 ± 7.9 vs. 10.8 ± 4.6 nmol/µmol, P = 0.015), leptin (21.1 ± 24.3 vs. 11.7 ± 18.6 ng/mL, P = 0.002), adiponectin (18.3 ± 7.1 vs. 15.6 ± 7.6 ng/mL, P = 0.007) and free T4 (16.2 ± 3.50 vs. 15.2 ± 3.53 pmol/L, P = 0.021). Vitamin D insufficiency (25(OH)D < 50 nmol/L) was found in 84.6% of females and 67.5% of males (P < 0.008) and SHPT (PTH > 6.8 pmol/L) in 43.2% and 25.3%, respectively (P = 0.016).
| Table 1. Demographic, Clinical and Laboratory Characteristics of the Studied Patients With Hip Fracture (n = 294) |
Associations between liver markers and parameters of mineral-bone metabolism, adiponectin, leptin and resistin, and clinical characteristics
In Pearson’s analyses (all biochemical variables log-transformed), after adjusting for age and sex, both serum GGT and ALT activities correlated negatively with serum OC levels (Table 2). GGT was associated positively with BAP, adiponectin and TSH and negatively with NTx/Cr, T4 and hemoglobin concentrations, while ALT correlated positively with T4. Serum ALP activity, as could be expected, demonstrated a strong correlation with BAP, and a significant positive association with both urinary resorption markers (DPD/Cr and NTx/Cr) and a negative correlation with 25(OH)D, T4 and hemoglobin. Albumin correlated with resistin and hemoglobin. Bilirubin was inversely associated with OC and positively with PTH, adiponectin and resistin. We calculated also the OC/BAP ratio as an index reflecting osteoblast differentiation/maturation [27]. Results showed significant inverse correlations between the OC/BAP ratio and all liver markers (GGT, ALT, ALP and bilirubin) except albumin.
| Table 2. Correlation of Serum Liver Markers With Indices of Bone-Mineral Metabolism, Leptin, Adiponectin, and Resistin Levels, Thyroid Markers and Hemoglobin |
Similar partial correlation analyses (after adjusting for age and sex) also demonstrated that serum GGT activity correlated with presence of CAD (β = 0.178, P = 0.003) and alcohol overuse defined as ≥ 3 times a week (β = 0.211, P < 0,001), ALT correlated negatively with Parkinson’s disease (PD, β = -0.173, P = 0.004), ALP with dementia (β = 0.118, P = 0.047), albumin was inversely associated with DM (β = -0.126, P = 0.032) and positively with hemoglobin (β = 0.281, P < 0.001), and bilirubin correlated with CAD (β = 0.142, P = 0.018) and atrial fibrillation (AF, β = 0.242, P < 0.001). There was a statistically significant inverse correlation between adiponectin and leptin (β = -0.196, P = 0.002), but there was no association between adiponectin and resistin (β = 0.043, P = 0.493), or between leptin and resistin (β = 009, P = 0.885).
When comparing patients with GGT < 30 U/L (median level) and GGT > 30 U/L, the latter group had, as would be expected, higher mean ALT (31.9 vs. 17.2 U/L, P = 0.004), BAP (29.6 vs. 24.2 U/L, P = 0.002), ALP (129.5 vs. 89.3 U/L, P < 0.001) and bilirubin (13.7 vs. 11.5 µmol/L, P = 0.011) levels, but also higher adiponectin (18.8 vs. 16.6 ng/mL, P = 0.015) and resistin concentrations (20.3 vs. 17.5 ng/mL, P = 0.038) and lower NTx/Cr urinary excretion (133.5 vs. 176.6 nmol/µmol, P = 0.048). Similar analyses for ALT < 20 U/L (median level) vs. > 20 U/L, bilirubin (< 20 µmol/L vs. > 20 µmol/L) and albumin (< 33 g/L vs. > 33 g/L) did not show significant associations either with any of the analyzed bone turnover markers, or with any of the adipokines (data not shown).
The patients were also stratified into two groups according to nutritional status: with (n = 99) and without malnutrition (n = 195). The first group showed significantly higher mean levels of urinary NTx/Cr (in women: 258.2 vs. 136.8 nmol/µmol, P < 0.001, in men: 134.5 vs. 102.6 nmol/µmol, P < 0.05), and serum adiponectin (19.9 vs. 17.3 ng/mL, P = 0.020, and 17.8 vs. 14.5 ng/mL, P = 0.05 in women and men, respectively). Liver markers, other mineral-bone parameters and resistin concentrations did not differ by nutritional status, in spite of the highly significant difference in mean leptin levels (3.1 vs. 30.4 ng/mL in women, 2.4 vs. 16.5 ng/mL in men, P < 0.001 for both groups). Malnourished men demonstrated the highest partial correlation between GGT and OC (r = - 0.497, P = 0.010), while the inverse correlation between GGT and OC/BAP ratio was similar and significant in all groups regardless of the nutritional status and gender (r = - 0.310 or higher, P < 0.004). Only in the malnourished women OC significantly correlated with adiponectin (r = 0.354, P = 0.006).
To further evaluate the association between OC and liver markers, adipokines and other indices of mineral-bone metabolism, we compared these parameters in HF patients, grouped according to serum OC levels. Patients with low OC levels (< 14 ng/mL, n = 156, or 53.2%), compared to the rest of the cohort (n = 138), were younger (80.7 ± 8.4 vs. 83.5 ± 7.7 years, P = 0.003), had higher mean serum GGT (67.3 ± 28.5 vs. 40.3 ± 21.2 U/L, P = 0.001) and 25(OH)D levels (40.8 ± 18.3 vs. 33.6 ± 17.1 nmol/L, P = 0.001), lower PTH (6.3 ± 4.3 vs. 7.7 ± 6.8 pmol/L, P = 0.034), calcium (2.26 ± 0.13 vs. 2.30 ± 0.11 mmol/L, P = 0.033), phosphate (0.88 ± 0.29 vs. 0.97 ± 0.28 mmol/L, P = 0.007), BAP (24.6 ± 12.6 vs. 28.4 ± 15.6 U/L, P = 0.027), and leptin (15.3 ± 18 vs. 22.1 ± 27.5 ng/mL, P = 0.013) levels, as well as lower both urinary bone resorption markers (DPD/Cr: 11.6 ± 5.7 vs. 13.7 ± 8.8 nmol/µmol, P = 0.024; NTx/Cr: 122.9 ± 119.6 vs. 204.2 ± 202.8 nmol/µmol, P < 0.001). No differences in mean values of ALT, ALP, bilirubin, magnesium, adiponectin, resistin and hemoglobin were observed.
Predictors of GGT, ALT, albumin and bilirubin (multiple regression analyses)
Multiple linear regression analyses with stepwise method were used to examine significant independent associations of liver markers with laboratory and clinical characteristics. In these models, each liver marker was set as dependent variable, while age, gender, OC, BAP, NTx/Cr, DPD/Cr, 25(OH)D, PTH, leptin, adiponectin, resistin, TSH, T4 and hemoglobin were set as independent variables (model 1). In model 2 further adjustment for alcohol use ≥ 3 drinks per week (yes/no), presence of CAD (yes/no), hypertension (yes/no), AF (yes/no), type 2 DM (yes/no), PD (yes/no) and CKD stage ≥ 3 (yes/no) was made. As presented in Table 3, reduced OC was an independent predictor of higher GGT, ALT and bilirubin, whereas higher BAP was associated with GGT and ALP. These analyses also showed that NTx/Cr, hemoglobin (both inversely), adiponectin, alcohol overuse and CAD (all three positively) were independent predictors of GGT activity. Higher ALP was predicted by elevated BAP and lower 25(OH)D levels. The independent predictors of serum albumin were hemoglobin and resistin. In the fully adjusted model, higher bilirubin levels were predicted by lower OC, higher adiponectin and presence of AF, although male gender, PTH and resistin were independent predictors in model 1 (included only laboratory parameters), suggesting that these variables are incorporated in clinical factors used in the model 2. The full panel of determinants predicted 27.0%, 7.8%, 23.3%, 11.8% and 13.2% of variance in serum GGT, ALT, ALP, albumin and bilirubin levels, respectively. The liver markers’ variance was only partially explained by the panel of determinants indicating the contribution of other regulating factors.
| Table 3. Multiple Linear Regression Analyses for Liver Markers in Older Patients With Hip Fracture |
To further explore these observations, we constructed similar multiple linear regression analyses to predict liver markers in patients with and without malnutrition. These revealed that only in malnourished women, OC was not an independent predictor of GGT or ALT, and NTx/Cr predicted ALP activity (β = 0.108, P = 0.042), while all other abovementioned correlations in the entire cohort were present regardless of the nutritional status.
Predictors of bone turnover markers (multiple regression analyses)
Results from linear multiple regression with each bone turnover marker as a dependent variable and age, sex, GGT, ALT, ALP, albumin, bilirubin, 25(OH)D, PTH, leptin, adiponectin and resistin (all biochemical variables were log-transformed) as independent variables are shown in Table 4. Five factors were significantly and independently associated with OC: age, ALP, leptin (all three positively), GGT and ALT (both negatively). BAP was independently predicted by GGT and ALP. Both elevated NTx/Cr and DPD/Cr were predicted by higher ALP activity and lower leptin concentrations; age also demonstrated a strong relationship with NTx/Cr. Finally, the independent determinants of OC/BAP ratio were GGT (inverse) and leptin (positive). Overall, the panel of determinants independently contributed to 15% of OC variance, to 20.4% of BAP, to 22.3% of NTx/Cr, to 13.5% of DPD/Cr, and to 11.6% of OC/BAP ratio variance.
| Table 4. Multiple Linear Regression Analyses for Bone Turnover Markers in Older Patients With Hip Fracture |
Multivariate regression modeling, using abovementioned laboratory and clinical variables and eGFR confirmed also a significant inverse bidirectional link between leptin and adiponectin (β = -0.331, P = 0.036 for adiponectin as an independent correlate of leptin, and β = -0.065, P = 0.036 for leptin as a predictor of adiponectin).
Clinical implications
From the practical point of view, we tried to evaluate the diagnostic value of hepatic markers as indicators of the mineral-bone metabolism status. We focused on GGT activity which seems to be more relevant to bone metabolism and performed logistic regression analyses with GGT as a dependent variable (GGT ≤ 20 U/L (cut-off level of the first tertile) vs. > 20 U/L). Among patients with the GGT > 20 U/L, there was a 2.3-fold increase of subjects with low circulating OC levels (< 14 ng/mL, lower limit of normal range), about a 4.8-fold increase of subjects with elevated of BAP activity (> 43 U/L, upper limit of normal range) and, consequently, a 2.6-fold increase of subjects with low OC/BAP ratio (< 0.6, median level). These associations remained significant after adjustments for age, sex, serum adiponectin, leptin and resistin levels, alcohol overuse, presence of CAD or DM (Table 5). Diagnostic value of GGT > 20 U/L was as follows: for presence of low OC (< 14 ng/mL) sensitivity 59.9%, specificity 60.2%, positive predictive value (PPV) 75.7%, negative predictive value (NPV) 42.1%, positive likelihood ratio (LR) 1.51; for high BAP (> 43 U/L) 9.5%, 97.8%, 90.0%, 34.6%, 4.41, respectively; for low OC/BAP ratio (< 0.6) 57.9%, 65.6%, 77.5%, 43.3%, 1.68, respectively. Although the diagnostic value was relatively low, this still would have translated in identifying about 76% of patients with abnormal bone formation status (PPV = 75.7% for low OC and PPV = 77.5% for low OC/BAP ratio) indicating that GGT > 20 U/L in the elderly can be used as a simple first step in detecting bone disease.
| Table 5. Serum Gamma-Glutamyltransferase Activity (GGT > 20 U/L) as a Predictor of Abnormal Bone Formation Markers in Older Patients With Hip Fracture |
Interestingly, GGT > 20 U/L was also associated with a two-fold increase of presence of CAD (OR: 1.94, 95% CI: 1.01 - 3.65, P = 0.050) (sensitivity 25.0%; specificity 85.6%, PPV 76.7%, NPV 37.6%, LR 1.73).
| Discussion | ▴Top |
The major findings of this cross-sectional study are the significant and independent associations and bidirectional links between liver markers, indices of bone and mineral metabolism and adipokines. Two main points arise from our results: 1) the liver-bone interactions, and 2) the role of adipokines in these relationships.
Liver-bone interactions
Our results are consistent with many studies that have shown that elevated liver markers are not only sensitive indicators of hepatobiliary diseases and alcohol consumption, but even within reference range, as in the present study, are associated with a variety of common diseases, such as CAD, AF, DM, dementia, PD [28-33], and mortality [34-37] independently of alcohol consumption. Reported in the literature age and gender-based differences in liver [32, 34-36] and bone turnover markers [38-42], as well as in three studied adipokines [43-46] were confirmed within our study population, specifically, the decrease of transaminase activities and increase of bone turnover (OC and NTx/Cr) with ageing, and higher levels of urinary bone resorption markers and circulating adiponectin and leptin in women than in men.
In the present study, we focused on associations between hepatic markers (in the majority of our patients, they were within the normal range), parameters of mineral and bone metabolism, and the concentrations of three adipokines. We found significant bidirectional links between liver and bone-mineral parameters, specifically between: 1) GGT and OC, NTx/Cr (both inverse) and BAP (positive), 2) ALT and OC (inverse), and 3) ALP and BAP (positive). In addition, ALP was an independent predictor of OC, NTx/Cr and DPD/Cr, and was negatively associated with 25(OH)D. OC was an independent negative correlate of bilirubin, and in the model which included only laboratory data, there was a significant association between PTH and bilirubin. Interestingly, the activity of ALT, a more specific hepatic marker than GGT, has shown similar to GGT inverse associations with OC but to a lesser degree.
Consistent with our findings, an inverse association of serum OC concentrations with ALT has been demonstrated in non-obese and obese subjects [13] and in patients with NAFLD [47, 48], independent of insulin resistance and the metabolic syndrome [47].
There are clinical and epidemiological reports, as well as in vitro and animal studies showing important physiological effects of GGT on bone metabolism. In postmenopausal women urinary GGT excretion exhibited a high correlation with DPD [49]. A large longitudinal study (16,036 Korean men aged ≥ 50 years) demonstrated that a higher serum GGT level was associated with increased development of osteoporotic fractures over a mean 3-year follow-up period [50]. Experimental data indicate that both a deficiency and an excess of GGT result in osteoporosis [49, 51-53]. GGT in vitro stimulates osteoclast formation (independently of its enzymatic activity) and expression of receptor activator of nuclear factor-kB ligand (RANKL) mRNA and protein from bone marrow stromal cells, and in transgenic mice promotes osteoporosis [51-53]. It has been proposed that osteopenia/osteoporosis in GGT-deficient (GGT-/+) mice is caused by suppression of bone formation, while excess of GGT results mainly in acceleration of bone resorption [53]. In our cohort of older HF patients, we found significant bidirectional inverse associations between GGT and both OC (an osteoblast-specific noncollagen protein, a bone formation marker) and NTx/Cr (a recognized bone resorption marker) and a positive association between GGT and BAP (a bone formation marker); GGT was an independent negative predictor of the OC/BAP ratio (an index of osteoblast differentiation) [27]. Our observations are in agreement with reports that OC and BAP are not uniformly associated. For example, BAP correlates positively with OC in healthy women and negatively with OC in women with liver disease [54]. OC is in a positive and BAP is in a negative relationship with serum insulin growth factor-I (GF-1) [27] and the OC/BAP ratio is inversely associated with the number of vertebral fractures. In the current study, the OC/BAP ratio adjusted for age and sex was significantly and negatively associated with GGT, ALT, ALP and bilirubin indicating that dysregulated osteoblast formation and differentiation is linked to liver status. Moreover, GGT was an independent negative predictor of the OC/BAP ratio. The opposite associations of GGT with two osteoblast-derived bone formation markers (OC and BAP) could point to its specific effect on osteoblast differentiation, as OC is a protein that binds to hydroxyapatite deposited in the matrix, whereas BAP is essential to the process of mineralization of the matrix after formation of osteoid [55, 56]. The negative correlation between GGT and NTx/Cr (in adjusted multivariate linear regression analyses higher serum GGT was a significant independent predictor of lower NTx/Cr, and vice versa) may reflect the complex role of GGT in coordinating the tightly coupled bone formation and resorption. It appears that GGT acts as a factor that maintains bone homeostasis, and plays dual role in physiopathology: higher GGT levels may affect osteoblast differentiation and decreases bone resorption. However, in malnourished women both serum OC and urine NTx/Cr were not independent predictors of GGT activity, while BAP was significantly associated with GGT (as it was observed in the total cohort and in non-malnourished subjects), suggesting that the links between liver markers and specific metabolic indices of bone remodeling are modulated by nutritional status and gender, and these may contribute to an individual’s propensity to osteoporosis and bone frailty.
The bidirectional link between BAP and ALP is not surprising as approximately half of circulating total ALP comes from bone [55, 56].
Our study also showed that serum hemoglobin is a significant and independent predictor of GGT (inverse association) and albumin, another determinant of bone health in the elderly [57]. These results are in agreement with previous epidemiologic studies that demonstrated that hemoglobin level is positively associated with risk of development of NAFLD independently of body mass index, type 2 DM, and other metabolic diseases [58]. Taken together, our results suggest reciprocal liver-bone cross-talk. Liver plays an important role in the regulation and dysregulation of bone remodeling and vice versa (bone-mineral metabolism parameters modulate various hepatic functions). In these interactions, GGT seems to be a major factor acting on and being influenced by bone turnover markers, as well as multiple other metabolic factors and conditions.
Adipokines and markers of hepatic, mineral and bone metabolism
Extensive studies of leptin, adiponectin, and resistin, the three best-studied adipokines, in both hepatic and bone metabolism have produced conflicting results, especially when comparing data from clinical and animal reports. In HF patients, studies on adipokines are limited [44, 59-62], and the relationship of adipokines with liver and bone turnover markers have not been evaluated systematically.
The present study, to our knowledge for the first time, documents the links between adiponectin, leptin and resistin, and liver and bone-mineral indices in patients with HF. In our multiple linear regression analyses, adiponectin, but not leptin and resistin, was an independent predictor of GGT activity, resistin (in humans, unlike in rodents, it is produced mostly in macrophages) [63] was a determinant for albumin and bilirubin (the latter in a model adjusted only for laboratory variables), whereas leptin independently predicted OC, OC/BAP ratio, NTx/Cr and DPD/Cr levels (inversely for both resorption markers).
In regard to adipokines-liver relationship, our observations are consistent with current evidence that the liver is a major target organ for many of its effects [18, 64]. In our study, as in previous reports, adiponectin was negatively associated with GGT [65, 66], although others found only a weak inverse association [67], or even a positive correlation [68] between these variables; some researchers demonstrated that adiponectin was negatively associated with circulating ALT activity [65-67, 69]. In a previous study, as in ours, serum levels of resistin positively correlated with bilirubin [68]. Our data are also in line with recent experiments showing that the majority of metabolic effects of leptin essential for energy homeostasis are dependent on its action in nonhepatocyte cells and/or the central nervous system [70].
Concerning the adipokine-bone links, we found that in the entire cohort leptin (but not adiponectin or resistin) was an independent predictor of OC, OC/BAP ratio and both bone resorption markers. These findings are in contrast with the widespread (based mainly on animal studies) view that leptin acting primarily through the centrally (hypothalamus and brainstem) mediated pathways suppresses bone formation [14, 71, 72], but are in accord with some, but not all [34, 44, 73-76], clinical studies that reported a positive association between serum leptin and OC levels [77, 78] and with experiments showing that peripheral leptin increases osteoblast proliferation, differentiation and activity, inhibits osteoclast activity and bone resorption, increasing bone mass [79-81]. Furthermore, a meta-analysis (59 studies) found that leptin was positively associated with bone mineral density (BMD) and high levels of leptin were predictive of lower risk of fractures [82].
There is considerable controversy on the role of adiponectin in bone metabolism. Although in vitro studies demonstrated that adiponectin stimulates the differentiation and mineralization of osteoblasts and the expression of OC, directly inhibits osteoclast activity and indirectly stimulates osteoclast differentiation [83-86], it is less clear whether adiponectin plays the same role in humans. We found that in malnourished women (but not in other groups) adiponectin significantly correlated with OC, suggesting that bone-adipocyte interaction in these subjects may be regulated through adiponectin and OC. Adiponectin was positively associated with total OC [87, 88] and BAP [89] in postmenopausal women and in Chinese men [90], but in other studies no correlation with any of bone formation or bone resorption markers was found [45, 91]. Several clinical studies have shown an inverse relationship between circulating adiponectin and BMD independent of gender and menopausal status, and some reviewers concluded that adiponectin may be a negative regulator of bone mass [82, 92-94], while other emphasized that the data on this association are inconsistent [95].
It is also uncertain whether resistin is related to bone turnover markers. Resistin is expressed in primary human bone marrow stem cells and in mature human osteoblasts [96], but no association between resistin and BMD was found in clinical studies [92]. In our study, circulating resistin levels in line with the latter data were not associated with bone turnover markers.
In line with many previous studies, we observed a significant inverse relationship between adiponectin and leptin, both by paired comparisons adjusted for age and sex, as well as multivariate regression modeling. This indicates that metabolic functions of leptin and adiponectin, which generally affect cellular behavior in an opposing manner, should not be considered in isolation, but rather as complementary. Our data suggest that the bone remodeling, as measured by bone formation and resorption markers, depends, at least partially, on the regulatory interaction between adiponectin and leptin which may exert opposite effects on bone metabolism through different pathways. In other words, the adiponectin-leptin link appears as an important mediator of the association between GGT and OC.
Our analysis also identified that in malnourished women, in contrast to subjects without such condition, adiponectin is not associated with GGT, but strongly correlates with OC. It appears that in different diseases and in particular conditions adiponectin may be regulated in the opposite directions and may exert opposite activities [97, 98]. At the molecular level, variations have been found at all sites of adiponectin action including different subtypes of adiponectin receptors operating in the liver and bones [94, 99, 100]. Our observations are in agreement with a recent animal study, which concluded that adiponectin has the ability to regulate the same function in two opposite manners depending on where it acts and it opposes leptin’s influence [101]. Our results show that adiponectin may exert a negative net effect on bone metabolism by increasing GGT and decreasing circulating leptin levels (inverse adiponectin-leptin relationship); on the other hand, OC and adiponectin have opposite effects on GGT. However, these relationships are not consistently maintained, but expressed differently under various pathophysiological conditions indicating the level of complexity of the homeostatic system(s). Specifically, in malnourished women increases in serum adiponectin, which is produced almost exclusively by adipocytes, are associated with higher OC levels and do not correlate with GGT.
Alterations in vitamin D-PTH status are factors substantially contributing to the high prevalence of osteoporosis and fractures in the elderly [1]. Although vitamin D insufficiency and elevated PTH levels were recorded in 79.7% and 38.2% of our patients, respectively, in multivariate analyses no significant correlations were detected between GGT and ALT activities, on the one hand, and 25(OH)D and PTH concentrations, on the other, suggesting that the vitamin D-PTH axis is not an independent determinant of the transaminase activities. Also, 25(OH)D and PTH were not independent predictors of bone turnover markers, indicating that vitamin D and PTH are not involved in the relationship of these liver enzymes with bone remodeling. We found positive correlations between 25(OH)D and ALP, and between PTH and bilirubin, which indicate that vitamin D insufficiency and SHPT may affect specific liver functions, as it was previously reported for the PTH-bilirubin association [102, 103].
Potential mechanisms: combining regulatory elements
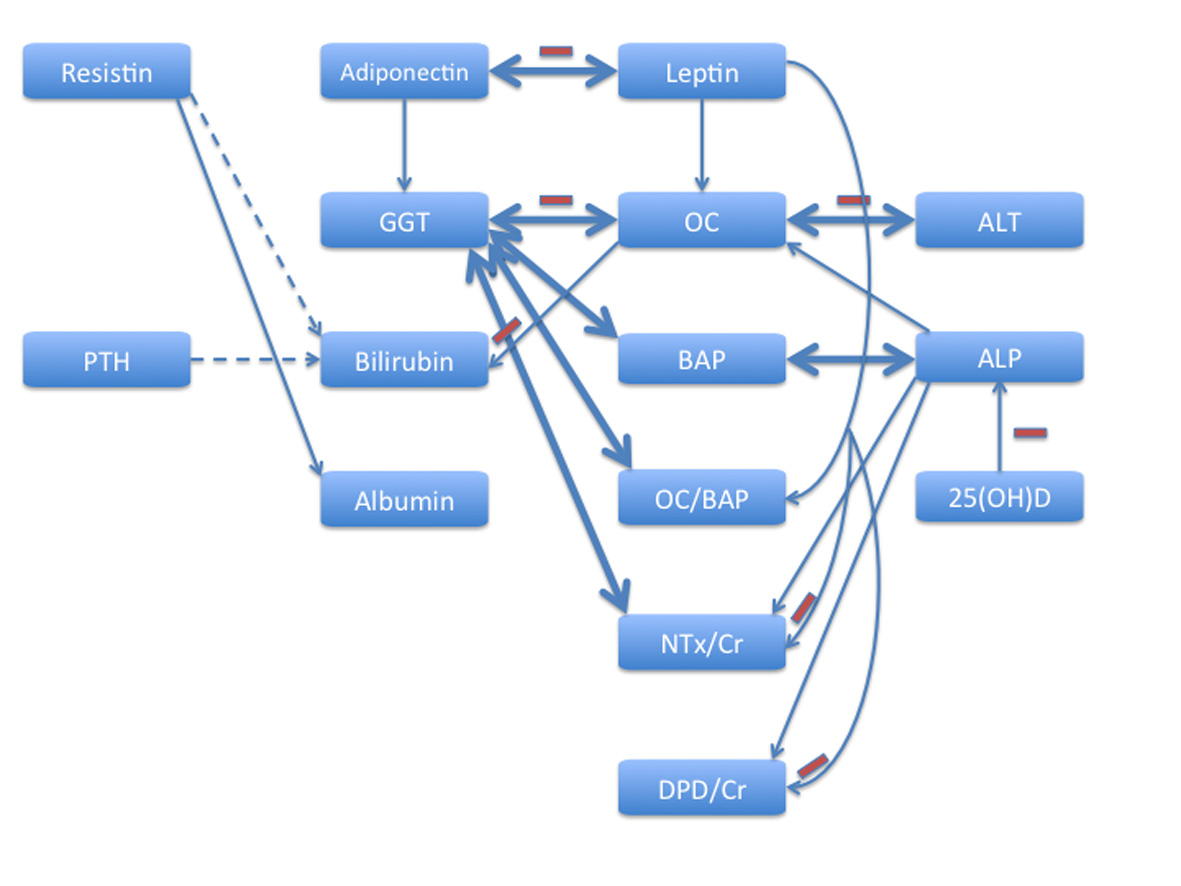
Our results illustrate complex interactions between different adipokines, liver function and mineral-bone metabolism, and extend this notion by showing these links in HF patients. The findings suggest liver function as an important component involved in bone remodeling. Significant independent correlates for OC included GGT, ALT (both negative), ALP, leptin and age (all three positive), for BAP-GGT and ALP, for NTx/Cr-GGT and leptin (both negative), ALP and age (both positive), for DPD/Cr-ALP (positive) and leptin (negative), and for OC/BAP ratio-GGT (negative) and leptin (positive). Integrating multidirectional interrelationship between adipokines, hepatic and bone metabolism, factors acting on and/or being influenced by each other, it is possible to propose a model that unifies a number of functions and observations that have been considered contradictory in the past (Fig. 1). This model based on the results of multivariate regression analyses takes into account that homeostasis necessitates reciprocal signaling between hepatic function, components of mineral-bone metabolism and adipokines. It should be viewed as a part of a complex network that integrates and orchestrates the liver-bone-adipose tissue axis in health and diseases. Of major pathophysiological interest is the feedback loop between GGT and OC, in which adiponectin and leptin appear as two important counterplayers: the former acts as a positive regulator of GGT, while the latter is a positive regulator of OC (Fig. 1, upper part). Importantly, these relationships are not invariant, but depend on multiple underlying conditions, among which the nutritional status and gender may be the key contributors.
The stronger (compared to other liver indices) and bidirectional association of GGT, an indicator of oxidative stress [28, 104], with bone turnover markers suggests that the liver-bone links might reflect systemic rather than solely hepatic processes. Several lines of evidence indicate oxidative stress, a systemic process implicated in the regulation of ageing and longevity [105], as well as numerous pathological conditions [106, 107], including liver diseases [108] and osteoporosis [109, 110], as a possible unifying factor. 1) GGT is responsible for the extracellular catabolism of the main antioxidant in mammalian cells - glutathione [28, 104, 111]. 2) The liver plays a key role in the systemic glutathione (GSH) homeostasis [112, 113]. 3) Adiponectin significantly contributes to oxidative damage [114]. 4) Oxidative stress produces deleterious effects in osteoblasts [115]. In addition, our study showed involvement of other factors which have also been proposed as indices of oxidant stress status, namely serum hemoglobin levels were significantly and independently associated with GGT activity (inversely) and albumin concentration (positively), while OC correlated with bilirubin level. Hemoglobin [116, 117], albumin [118-120] and bilirubin [118, 121] exhibit potent antioxidant properties. Increases in GGT activity may reflect the responses of counteracting mechanisms to protect against oxidative damage. Our findings suggest the need to explore in depth the role of liver function in bone metabolism, in particular, the participation of GGT in these processes.
Potential clinical implications
Of practical interest is the finding that in the HF patients GGT > 20 U/L (above the cut-off level of the first tertile) corresponds with a two times higher prevalence of CAD, low OC levels (< 14 ng/mL, lower limit of normal range) and 2.7 times higher prevalence of low OC/BAP ratio. Consistent with our results, previous studies have found GGT to be associated with CAD [31, 33, 122, 123]. Collectively these results indicate that GGT within the normal range is broadly linked to health conditions, including osteoblast dysfunction, and older patients with GGT > 20 U/L need an examination of their bone status and consideration of an antiosteoporotic medication with anabolic properties. Currently, osteoporosis is predominantly treated with antiresorptive medications, despite the fact that in a significant proportion of elder patients bone loss is primarily attributed to the impaired osteoblastic activity [124]. Serum GGT > 20 U/L may be a promising indirect marker useful as the first and easy step in diagnostic evaluation of impaired bone metabolism in the elderly and pointing to the need of individualized therapy; its PPV for low OC and for low OC/BAP ratio is above 75%. The clinical significance of this marker should be confirmed and validated by longitudinal studies.
Limitations
The main limitations of this study are the cross-sectional nature of the analysis and the single point-in-time assessment of the hepatic indices, mineral-bone turnover markers and adipokines. Therefore, the associations between the variables found in the study suggest a link, but a causal relationship cannot be established. Secondly, we did not measure the undercarboxylated OC (unOC), RANKL, osteoprotegerin, high molecular weight adiponectin, and sexual hormones, each of which may be involved in the liver-bone interaction. Thirdly, we could not eliminate the possible effect of medications used on the present findings, although we attempted to control most potential confounders. Finally, because the study population consisted predominantly of Caucasians, generalization of the results should be done with caution. The strengths of our study include the use of data from an unselected well-characterized cohort of HF patients, simultaneous measurements of multiple hepatic, mineral-bone biomarkers and three adipokines. Pearson’s correlation coefficients preserved statistical significance after Bonferroni and Sidak adjustments. The variance inflation factor in our multivariate regression analyses ranged between 1.07 and 1.32 indicating that the multicollinearity phenomena were not significant.
Conclusions
In older HF patients liver functions (within normal range in the vast majority) are associated with indices of bone metabolism. The complex liver-bone-adipokine interactions include bidirectional links between GGT and markers of bone formation (OC, BAP), differentiation (OC/BAP ratio) and resorption (NTx/Cr), as well as between ALT and OC. Adiponectin is an independent predictor of GGT, whereas leptin is a determinant of OC and bone resorption markers; these relationships are modulated by nutritional status and gender. GGT > 20 U/L is associated with a two times higher prevalence of low OC levels and 2.7 times higher prevalence of low OC/BAP ratio; it may be used as a marker of impaired bone metabolism.
Acknowledgement
We thank Wichat Srikusalanukul, MD, for help with the statistical analyses.
Financial Support
None.
Potential Competing Interests
None.
Grant Support
None.
| References | ▴Top |
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4-8.
doi pubmed - Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25(4):531-541.
doi pubmed - D'Amour P. Acute and chronic regulation of circulating PTH: significance in health and in disease. Clin Biochem. 2012;45(12):964-969.
doi pubmed - Fisher L, Byrnes E, Fisher AA. Prevalence of vitamin K and vitamin D deficiency in patients with hepatobiliary and pancreatic disorders. Nutr Res. 2009;29(9):676-683.
doi pubmed - Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5(4):513-520.
doi pubmed - Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125(3):941-966.
doi - Collier J. Bone disorders in chronic liver disease. Hepatology. 2007;46(4):1271-1278.
doi pubmed - Luxon BA. Bone disorders in chronic liver diseases. Curr Gastroenterol Rep. 2011;13(1):40-48.
doi pubmed - Patti A, Gennari L, Merlotti D, Dotta F, Nuti R. Endocrine actions of osteocalcin. Int J Endocrinol. 2013;2013:846480.
doi pubmed - Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28(1):52-64.
doi pubmed - Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456-469.
doi pubmed - Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310(1-2):21-29.
doi pubmed - Fernandez-Real JM, Ortega F, Gomez-Ambrosi J, Salvador J, Fruhbeck G, Ricart W. Circulating osteocalcin concentrations are associated with parameters of liver fat infiltration and increase in parallel to decreased liver enzymes after weight loss. Osteoporos Int. 2010;21(12):2101-2107.
doi pubmed - Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54(6):1291-1297.
doi pubmed - Schwetz V, Pieber T, Obermayer-Pietsch B. The endocrine role of the skeleton: background and clinical evidence. Eur J Endocrinol. 2012;166(6):959-967.
doi pubmed - Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014;561:137-146.
doi pubmed - Tsochatzis E, Papatheodoridis GV, Archimandritis AJ. The evolving role of leptin and adiponectin in chronic liver diseases. Am J Gastroenterol. 2006;101(11):2629-2640.
doi pubmed - Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50(3):957-969.
doi pubmed - Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129-139.
doi pubmed - Chiarugi P, Fiaschi T. Adiponectin in health and diseases: from metabolic syndrome to tissue regeneration. Expert Opin Ther Targets. 2010;14(2):193-206.
doi pubmed - Bluher M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3(3):230-240.
doi pubmed - Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best Pract Res Clin Endocrinol Metab. 2014;28(1):119-130.
doi pubmed - Mather KJ, Goldberg RB. Clinical use of adiponectin as a marker of metabolic dysregulation. Best Pract Res Clin Endocrinol Metab. 2014;28(1):107-117.
doi pubmed - Fisher AA, Srikusalanukul W, Davis MW, Smith PN. Clinical profiles and risk factors for outcomes in older patients with cervical and trochanteric hip fracture: similarities and differences. J Trauma Manag Outcomes. 2012;6(1):2.
doi pubmed - Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622-627.
doi pubmed - Bouillanne O, Golmard JL, Coussieu C, Noel M, Durand D, Piette F, Nivet-Antoine V. Leptin a new biological marker for evaluating malnutrition in elderly patients. Eur J Clin Nutr.2007; 61(5):647-654.
doi pubmed - Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Serum osteocalcin/bone-specific alkaline phosphatase ratio is a predictor for the presence of vertebral fractures in men with type 2 diabetes. Calcif Tissue Int. 2009;85(3):228-234.
doi pubmed - Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263-355.
doi pubmed - Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M, Blomhoff R, et al. Gamma-glutamyltransferase and diabetes--a 4 year follow-up study. Diabetologia. 2003;46(3):359-364.
pubmed - Meisinger C, Doring A, Schneider A, Lowel H. Serum gamma-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis. 2006;189(2):297-302.
doi pubmed - Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27(12):2729-2735.
doi pubmed - Dong MH, Bettencourt R, Brenner DA, Barrett-Connor E, Loomba R. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clin Gastroenterol Hepatol. 2012;10(3):285-290 e281.
- Weikert C, Drogan D, di Giuseppe R, Fritsche A, Buijsse B, Nothlings U, Willich SN, et al. Liver enzymes and stroke risk in middle-aged German adults. Atherosclerosis. 2013;228(2):508-514.
doi pubmed - Elinav E, Ackerman Z, Maaravi Y, Ben-Dov IZ, Ein-Mor E, Stessman J. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc. 2006;54(11):1719-1724.
doi pubmed - Le Couteur DG, Blyth FM, Creasey HM, Handelsman DJ, Naganathan V, Sambrook PN, Seibel MJ, et al. The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci. 2010;65(7):712-717.
doi pubmed - Ruhl CE, Everhart JE. The association of low serum alanine aminotransferase activity with mortality in the US population. Am J Epidemiol. 2013;178(12):1702-1711.
doi pubmed - Liu Z, Ning H, Que S, Wang L, Qin X, Peng T. Complex association between alanine aminotransferase activity and mortality in general population: a systematic review and meta-analysis of prospective studies. PLoS One. 2014;9(3):e91410.
doi pubmed - Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, Dooley KC, et al. Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem. 2009;42(10-11):929-942.
doi pubmed - Eastell R, Garnero P, Audebert C, Cahall DL. Reference intervals of bone turnover markers in healthy premenopausal women: results from a cross-sectional European study. Bone. 2012;50(5):1141-1147.
doi pubmed - Michelsen J, Wallaschofski H, Friedrich N, Spielhagen C, Rettig R, Ittermann T, Nauck M, et al. Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone. 2013;57(2):399-404.
doi pubmed - Niemann I, Hannemann A, Nauck M, Spielhagen C, Volzke H, Wallaschofski H, Friedrich N. The association between insulin-like growth factor I and bone turnover markers in the general adult population. Bone. 2013;56(1):184-190.
doi pubmed - Hannemann A, Breer S, Wallaschofski H, Nauck M, Baumeister SE, Barvencik F, Amling M, et al. Osteocalcin is associated with testosterone in the general population and selected patients with bone disorders. Andrology. 2013;1(3):469-474.
doi pubmed - Weiss LA, Barrett-Connor E, von Muhlen D, Clark P. Leptin predicts BMD and bone resorption in older women but not older men: the Rancho Bernardo study. J Bone Miner Res. 2006;21(5):758-764.
doi pubmed - Shabat S, Nyska M, Eintacht S, Lis M, Bogomolni A, Berner Y, Kestanbaum-Shainkin R. Serum leptin level in geriatric patients with hip fractures: possible correlation to biochemical parameters of bone remodeling. Arch Gerontol Geriatr. 2009;48(2):250-253.
doi pubmed - Sodi R, Hazell MJ, Durham BH, Rees C, Ranganath LR, Fraser WD. The circulating concentration and ratio of total and high molecular weight adiponectin in post-menopausal women with and without osteoporosis and its association with body mass index and biochemical markers of bone metabolism. Clin Biochem. 2009;42(13-14):1375-1380.
doi pubmed - Andreasson AN, Unden AL, Elofsson S, Brismar K. Leptin and adiponectin: distribution and associations with cardiovascular risk factors in men and women of the general population. Am J Hum Biol. 2012;24(5):595-601.
doi pubmed - Yilmaz Y, Kurt R, Eren F, Imeryuz N. Serum osteocalcin levels in patients with nonalcoholic fatty liver disease: association with ballooning degeneration. Scand J Clin Lab Invest. 2011;71(8):631-636.
doi pubmed - Liu JJ, Chen YY, Mo ZN, Tian GX, Tan AH, Gao Y, Yang XB, et al. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in adult males, South China. Int J Mol Sci. 2013;14(10):19782-19791.
doi pubmed - Asaba Y, Hiramatsu K, Matsui Y, Harada A, Nimura Y, Katagiri N, Kobayashi T, et al. Urinary gamma-glutamyltransferase (GGT) as a potential marker of bone resorption. Bone. 2006;39(6):1276-1282.
doi pubmed - Kim BJ, Baek S, Ahn SH, Kim SH, Jo MW, Bae SJ, Kim HK, et al. A higher serum gamma-glutamyl transferase level could be associated with an increased risk of incident osteoporotic fractures in Korean men aged 50 years or older. Endocr J. 2014;61(3):257-263.
doi pubmed - Levasseur R, Barrios R, Elefteriou F, Glass DA, 2nd, Lieberman MW, Karsenty G. Reversible skeletal abnormalities in gamma-glutamyl transpeptidase-deficient mice. Endocrinology. 2003;144(7):2761-2764.
doi pubmed - Niida S, Kawahara M, Ishizuka Y, Ikeda Y, Kondo T, Hibi T, Suzuki Y, et al. Gamma-glutamyltranspeptidase stimulates receptor activator of nuclear factor-kappaB ligand expression independent of its enzymatic activity and serves as a pathological bone-resorbing factor. J Biol Chem. 2004;279(7):5752-5756.
doi pubmed - Hiramatsu K, Asaba Y, Takeshita S, Nimura Y, Tatsumi S, Katagiri N, Niida S, et al. Overexpression of gamma-glutamyltransferase in transgenic mice accelerates bone resorption and causes osteoporosis. Endocrinology. 2007;148(6):2708-2715.
doi pubmed - Steinberg KK, Bonkovsky HL, Caudill SP, Bernhardt RK, Hawkins M. Osteocalcin and bone alkaline phosphatase in the serum of women with liver disease. Ann Clin Lab Sci. 1991;21(5):305-314.
pubmed - Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996;17(4):333-368.
pubmed - Gundberg CM, Looker AC, Nieman SD, Calvo MS. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone. 2002;31(6):703-708.
doi - Nakamura K, Oyama M, Saito T, Oshiki R, Kobayashi R, Nishiwaki T, Nashimoto M, et al. Nutritional and biochemical parameters associated with 6-year change in bone mineral density in community-dwelling Japanese women aged 69 years and older: The Muramatsu Study. Nutrition. 2012;28(4):357-361.
doi pubmed - Yu C, Xu C, Xu L, Yu J, Miao M, Li Y. Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J Hepatol. 2012;56(1):241-247.
doi pubmed - Di Monaco M, Vallero F, Di Monaco R, Mautino F, Cavanna A. Fat body mass, leptin and femur bone mineral density in hip-fractured women. J Endocrinol Invest. 2003;26(12):1180-1185.
doi pubmed - Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, Kanaya AM, et al. Adipokines and the risk of fracture in older adults. J Bone Miner Res. 2011;26(7):1568-1576.
doi pubmed - Fisher AA, Goh SL, Srikusalankul W, Southcott EN, Davis MW. Serum leptin levels in older patients with hip fracture--impact on peri-operative myocardial injury. Am Heart Hosp J. 2009;7(1):9-16.
doi pubmed - Fisher A, Srikusalanukul W, Davis M, Smith P. Interactions between Serum Adipokines and Osteocalcin in Older Patients with Hip Fracture. Int J Endocrinol. 2012;2012:684323.
doi pubmed - Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300(2):472-476.
doi - Musso G, Paschetta E, Gambino R, Cassader M, Molinaro F. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol Med. 2013;19(9):522-535.
doi pubmed - Lopez-Bermejo A, Botas P, Funahashi T, Delgado E, Kihara S, Ricart W, Fernandez-Real JM. Adiponectin, hepatocellular dysfunction and insulin sensitivity. Clin Endocrinol (Oxf). 2004;60(2):256-263.
doi - Targher G, Bertolini L, Scala L, Poli F, Zenari L, Falezza G. Decreased plasma adiponectin concentrations are closely associated with nonalcoholic hepatic steatosis in obese individuals. Clin Endocrinol (Oxf). 2004;61(6):700-703.
doi pubmed - Mochizuki K, Misaki Y, Miyauchi R, Takabe S, Shimada M, Ichikawa Y, Goda T. Associations between markers of liver injury and cytokine markers for insulin sensitivity and inflammation in middle-aged Japanese men not being treated for metabolic diseases. J Nutr Sci Vitaminol (Tokyo). 2011;57(6):409-417.
doi - Durazzo M, Niro G, Premoli A, Morello E, Rizzotto ER, Gambino R, Bo S, et al. Type 1 autoimmune hepatitis and adipokines: new markers for activity and disease progression? J Gastroenterol. 2009;44(5):476-482.
doi pubmed - Lu JY, Su TC, Liu YH, Hsu HJ, Chen CL, Yang WS. Lower plasma adiponectin is correlated to higher alanine aminotransferase independent of metabolic factors and hepatitis B virus carrier status. Intern Med J. 2007;37(6):365-371.
doi pubmed - Cortes VA, Cautivo KM, Rong S, Garg A, Horton JD, Agarwal AK. Leptin ameliorates insulin resistance and hepatic steatosis in Agpat2-/- lipodystrophic mice independent of hepatocyte leptin receptors. J Lipid Res. 2014;55(2):276-288.
doi pubmed - Driessler F, Baldock PA. Hypothalamic regulation of bone. J Mol Endocrinol. 2010;45(4):175-181.
doi pubmed - Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42(5):837-840.
doi pubmed - Hipmair G, Bohler N, Maschek W, Soriguer F, Rojo-Martinez G, Schimetta W, Pichler R. Serum leptin is correlated to high turnover in osteoporosis. Neuro Endocrinol Lett. 2010;31(1):155-160.
pubmed - Roux C, Arabi A, Porcher R, Garnero P. Serum leptin as a determinant of bone resorption in healthy postmenopausal women. Bone. 2003;33(5):847-852.
doi pubmed - Schett G, Kiechl S, Bonora E, Redlich K, Woloszczuk W, Oberhollenzer F, Jocher J, et al. Serum leptin level and the risk of nontraumatic fracture. Am J Med. 2004;117(12):952-956.
doi pubmed - Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24(5):785-791.
doi pubmed - Berry PA, Jones SW, Cicuttini FM, Wluka AE, Maciewicz RA. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011;63(3):700-707.
doi pubmed - Aoki A, Muneyuki T, Yoshida M, Munakata H, Ishikawa SE, Sugawara H, Kawakami M, et al. Circulating osteocalcin is increased in early-stage diabetes. Diabetes Res Clin Pract. 2011;92(2):181-186.
doi pubmed - Thomas T. Leptin and fragility fracture: evidence for a protective effect in humans. Am J Med. 2004;117(12):966-968.
doi pubmed - Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28(1):22-34.
doi pubmed - Naot D, Cornish J. Cytokines and Hormones That Contribute to the Positive Association between Fat and Bone. Front Endocrinol (Lausanne). 2014;5:70.
- Biver E, Salliot C, Combescure C, Gossec L, Hardouin P, Legroux-Gerot I, Cortet B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(9):2703-2713.
doi pubmed - Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150(8):3603-3610.
doi pubmed - Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007;8:51.
doi pubmed - Wang QP, Yang L, Li XP, Xie H, Liao EY, Wang M, Luo XH. Effects of 17beta-estradiol on adiponectin regulation of the expression of osteoprotegerin and receptor activator of nuclear factor-kappaB ligand. Bone. 2012;51(3):515-523.
doi pubmed - Lin YY, Chen CY, Chuang TY, Lin Y, Liu HY, Mersmann HJ, Wu SC, et al. Adiponectin receptor 1 regulates bone formation and osteoblast differentiation by GSK-3beta/beta-catenin signaling in mice. Bone. 2014;64:147-154.
doi pubmed - Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T. Adiponectin is associated with changes in bone markers during glycemic control in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(8):3031-3037.
doi pubmed - Kanazawa I, Yamaguchi T, Tada Y, Yamauchi M, Yano S, Sugimoto T. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone. 2011;48(4):720-725.
doi pubmed - Wu N, Wang QP, Li H, Wu XP, Sun ZQ, Luo XH. Relationships between serum adiponectin, leptin concentrations and bone mineral density, and bone biochemical markers in Chinese women. Clin Chim Acta. 2010;411(9-10):771-775.
doi pubmed - Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta. 2008;387(1-2):31-35.
doi pubmed - Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obes (Lond). 2010;34(5):852-858.
doi pubmed - Magni P, Dozio E, Galliera E, Ruscica M, Corsi MM. Molecular aspects of adipokine-bone interactions. Curr Mol Med. 2010;10(6):522-532.
pubmed - Ruscica M, Steffani L, Magni P. Adiponectin interactions in bone and cartilage biology and disease. Vitam Horm. 2012;90:321-339.
doi pubmed - Kanazawa I. Adiponectin in metabolic bone disease. Curr Med Chem. 2012;19(32):5481-5492.
doi pubmed - Lubkowska A, Dobek A, Mieszkowski J, Garczynski W, Chlubek D. Adiponectin as a biomarker of osteoporosis in postmenopausal women: controversies. Dis Markers. 2014;2014:975178.
doi pubmed - Thommesen L, Stunes AK, Monjo M, Grosvik K, Tamburstuen MV, Kjobli E, Lyngstadaas SP, et al. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem. 2006;99(3):824-834.
doi pubmed - Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64(1):1-10.
doi pubmed - Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17(23):2801-2811.
pubmed - Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 2008;32(Suppl 7):S13-18.
doi pubmed - Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28(1):15-23.
doi pubmed - Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013;17(6):901-915.
doi pubmed - Kirch W, Hofig M, Ledendecker T, Schmidt-Gayk H. Parathyroid hormone and cirrhosis of the liver. J Clin Endocrinol Metab. 1990;71(6):1561-1566.
doi pubmed - Mahdy KA, Ahmed HH, Mannaa F, Abdel-Shaheed A. Clinical benefits of biochemical markers of bone turnover in Egyptian children with chronic liver diseases. World J Gastroenterol. 2007;13(5):785-790.
doi pubmed - Lee DH, Blomhoff R, Jacobs DR, Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38(6):535-539.
doi pubmed - Harman D. Origin and evolution of the free radical theory of aging: a brief personal history, 1954-2009. Biogerontology. 2009;10(6):773-781.
doi pubmed - Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46(5-6):241-281.
doi pubmed - Edrey YH, Salmon AB. Revisiting an age-old question regarding oxidative stress. Free Radic Biol Med. 2014;71:368-378.
doi pubmed - Cichoz-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082-8091.
doi pubmed - Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369-374.
doi pubmed - Abdollahi M, Moridani MY, Aruoma OI, Mostafalou S. Oxidative stress in aging. Oxid Med Cell Longev. 2014;2014:876834.
doi pubmed - Yamada J, Tomiyama H, Yambe M, Koji Y, Motobe K, Shiina K, Yamamoto Y, et al. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis. 2006;189(1):198-205.
doi pubmed - Ookhtens M, Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin Liver Dis. 1998;18(4):313-329.
doi pubmed - Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143-3153.
doi pubmed - Fukushima J, Kamada Y, Matsumoto H, Yoshida Y, Ezaki H, Takemura T, Saji Y, et al. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol Res. 2009;39(7):724-738.
doi pubmed - Almeida M, O'Brien CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci. 2013;68(10):1197-1208.
doi pubmed - Liu W, Baker SS, Baker RD, Nowak NJ, Zhu L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS One. 2011;6(9):e24363.
doi pubmed - Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. Am J Gastroenterol. 2013;108(8):1299-1304.
doi pubmed - Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59(9-10):945-952.
pubmed - Anraku M, Chuang VT, Maruyama T, Otagiri M. Redox properties of serum albumin. Biochim Biophys Acta. 2013;1830(12):5465-5472.
doi pubmed - Griffiths HR, Dias IH, Willetts RS, Devitt A. Redox regulation of protein damage in plasma. Redox Biol. 2014;2:430-435.
doi pubmed - Jansen T, Daiber A. Direct Antioxidant Properties of Bilirubin and Biliverdin. Is there a Role for Biliverdin Reductase? Front Pharmacol. 2012;3:30.
doi pubmed - Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112(14):2130-2137.
doi pubmed - Long Y, Zeng F, Shi J, Tian H, Chen T. Gamma-glutamyltransferase predicts increased risk of mortality: a systematic review and meta-analysis of prospective observational studies. Free Radic Res. 2014;48(6):716-728.
doi pubmed - Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10(2):191-197.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.