| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 2, Number 1, February 2012, pages 1-10
Apparent Sex-Specific Divergence of Acylation Stimulating Protein Levels With Respect to Metabolic Parameters of Pathogenetic and Clinical Relevance
Reza Rezvania, Altan Onatb, c, Gunay Cand, Katherine Cianflonea, e
aCentre de Recherche Institut Universitaire de Cardiologie et Pneumologie de Quebec, Laval University, Quebec, Canada
bTurkish Society of Cardiology, Turkey
cDepartment of Cardiology, Cerrahpasa Medical Faculty, Istanbul University, Turkey
dDepartment of Public Health, Cerrahpasa Medical Faculty, Istanbul University, Istanbul, Turkey
eCorresponding author: Katherine Cianflone, Centre de Recherche IUCPQ, Universite Laval, Y4332, 2725 Chemin Ste-Foy, Quebec, QC, G1V 4G5, Canada
Manuscript accepted for publication February 3, 2012
Short title: ASP and Metabolic Parameters
doi: https://doi.org/10.4021/jem58w
| Abstract | ▴Top |
Background: Acylation stimulating protein (ASP) is an adipose tissue-derived hormone that regulates triglyceride (TG) synthesis and glucose transport. Associations of ASP and/or its precursor complement C3 have been demonstrated with obesity, insulin resistance, diabetes, and cardiovascular diseases. We determined fasting serum ASP in a Turkish adult population sample and assessed relationships with cardiometabolic risk factors.
Methods: Cross-sectional population-based study recruiting 224 men and women from the Turkish Adult Risk Factor (TARF) study.
Results: Geometric Mean ASP levels (median) in women (271 nmol/L) tended to be lower than men (305 nmol/L) (P = 0.059), and this was also true in most subgroups with vs. without cardiometabolic disorders. Interestingly, correlations of ASP diverged in direction across genders with TG, glucose, height, age and other risk variables.
Conclusion: Gender divergence is an aspect of this Turkish population that has been noted for various lipid parameters (HDL, Lp(a)), association of cardiometabolic risk with smoking and alcohol intake and the response of the pro-inflammatory state to adiposity. This is consistent in the present study, where metabolic states correlate with ASP, but are divergent between genders.
Keywords: Obesity; ASP; Cardiometabolic disorders
| Introduction | ▴Top |
Cardiometabolic risk is a constellation of metabolic and underlying risk factors that significantly increase an individual’s risk of having a cardiovascular event or developing metabolic abnormalities such as type 2 diabetes (T2D). Metabolic syndrome (MetS) has been considered as a specific subset of cardiometabolic risks that, when clustered together, impart a relative increase in risk of cardiovascular disease (CVD) [1]. Approximately 9%, 21%, and 35% of normal weight, overweight, and obese adolescents [2] and 23.5%, 48.7% and 68.3% of normal weight, overweight, and obese adults respectively [3] have cardiometabolic risk factor clustering in U.S. The prevalence of metabolic syndrome (MetS) is approximately 34.6% in the United States, 17.8 - 34.0% in Europe and 12.8 - 41.1% in Asia [4]. Various studies based on the results of National Health and Nutrition Examination (NHANES) [2, 3] indicate that over a 10- to 15- year period, the prevalence of obesity, metabolic syndrome (MetS), and diabetes has increased by 34%, 48%, and 19% respectively. Obese individuals are characterized by a state of chronic low-grade inflammation [5] that may be causal in the development of insulin resistance and other disorders associated with obesity, such as hyperlipidemia, metabolic syndrome, or atherosclerosis [5].
Recently, adipose tissue has been recognized as a rich source of hormones (adipokines), some of which are pro-inflammatory and anti-inflammatory mediators [6]. Alteration of adipose tissue function, including modified adipokine secretion, plays a key role in the pathogenesis of obesity and metabolic disorders [7]. Acylation Stimulating Protein (ASP, aka C3adesArg) is one candidate, potentially contributing to the etiology of metabolic syndrome and cardiovascular disorders [8]. ASP is an adipokine generated by the ordered interaction of complement C3, factor B, and adipsin through activation of the alternative complement pathway, all produced by adipocytes (ASP review [9]). However, ASP/C3adesArg is also generated systemically following pro-inflammatory immune activation [8].
The main known roles of ASP are stimulation of free fatty acid incorporation into adipose tissue by increasing triglyceride synthesis and storage [9], increase in glucose uptake through enhanced translocation of glucose transporters [9] and reduction of triglyceride lipolysis in adipocytes through inhibition of hormone sensitive lipase [9]. The autocrine effect of ASP in human adipose tissue was shown to be mediated through binding to its receptor, C5L2, mediated through activation of protein kinase C, PI3kinase and Akt [10].
Several studies have shown that fasting ASP levels are increased in subjects with obesity [11, 12], insulin resistance [13], and type 2 diabetes (T2D) [14, 15]. These studies strongly support the idea that obesity and/or insulin resistance are associated with increased ASP levels [16]. However, higher ASP values were also observed in several metabolic disorders including cardiovascular disease [17], polycystic ovary syndrome [18], renal disease [19], nonalcoholic steatotic hepatitis [13] and dyslipidemia [9], without necessarily being associated with obesity and/or insulin resistance.
The prevalence of obesity and its consequences are rapidly increasing in developing countries [20]. Obesity and abdominal obesity are major and growing problems for Turkish adults, especially for Turkish women [20]. The Turkish population is known for the following characteristics: 1) The prevalence of obesity in the adult Turkish population (20.6% in men and 39.9% in women) is higher than most Western European countries (10 - 25% in Europe) and comparable with the United States (29% in white men and 50% in black women) [20, 21]. 2) The prevalence of obesity is similar in rural and urban areas in Turkey [20]. 3) In the Turkish population there is a high prevalence of cardiovascular risk factors including metabolic syndrome components (Onat A, review [22]). Consequently, there is an increased rate of cardiovascular disease and type 2 diabetes [22]. 4) Studies suggest that prevalence of abdominal obesity, MetS, and T2D are as great among Turkish women as men [23, 24]. 5) Adult Turks are recognized to generally have lower levels of plasma cholesterol and higher levels of triglyceride (TG) than Westerners [22]. The Turkish Adult Risk Factor (TARF) study, an established prospective population-based study, has contributed novel information on characterization of this population, including disorders with systemic inflammation [22].
The aims of this study were: to evaluate the adipokine ASP, in a large sample from a homogenous Turkish population and to evaluate the relationship of this adipokine with cardiometabolic risk factors in men as compared to women.
| Materials and Methods | ▴Top |
Sample population
Two hundred and twenty-four adult men and women were recruited randomly from participants of the 2005 - 2008 follow-up surveys of the TARF Study, a prospective study on cardiac disease and its risk factors in a representative sample of adults in Turkey, carried out biennially since 1990 in 59 communities throughout 7 geographical regions of the country [22]. Samples from available deep-frozen sera were randomly selected for study, without knowledge of any clinical or biochemical data. The study was approved by the Ethics Committee of the Medical Faculty, Istanbul University. Written informed consent for participation was obtained from all individuals. Data were obtained on medical and familial history via a questionnaire, physical examination, and blood samples.
Measurement of risk factors
Blood pressure (BP) was measured with an aneroid sphygmomanometer (Erka, Germany) in the sitting position on the right arm, and the mean of two recordings 3 min apart was recorded. Waist circumference was measured with the subject standing and wearing only underwear, at the level midway between the lower rib margin and the iliac crest. Blood samples were collected, after an overnight fast >11 hours, spun at 1000 g and shipped on cooled gel packs to Istanbul to be stored at -75 °C, until analyzed in a central laboratory. Serum concentrations of apolipoprotein apoB, apoA-I and C-reactive protein (CRP) were measured by nephelometry (BN Prospec, Behring Diagnostics, Westwood, MA). Plasma fibrinogen levels were assayed by modified Clauss method using Behring Fibrinometer II coagulometer and multifibren U kit. Serum concentrations of triglycerides and glucose, measured in the fasting state, as well as of total, low density lipoprotein (LDL) and high density lipoprotein (HDL) cholesterol (LDL-C, HDL-C plus 2nd generation, direct quantification) were determined using enzymatic kits from Roche Diagnostics with a Hitachi 902 autoanalyzer.
Fasting concentrations of sex hormone-binding globulin (SHBG) and insulin were assayed by electrochemiluminescence immunoassay (ECLIA) on Roche Elecsys 2010 immunautoanalyzer (Roche Diagnostics, Mannheim, Germany). ASP concentration was measured using an in-house sandwich ELISA, following previously published methodology [25].
Definitions
Atherogenic dyslipidemia was defined as TG > 1.7 mmol/L and HDL-C ≤ 1.03 mmol/L [26]. MetS was positive when 3 of 5 criteria of the National Cholesterol Education Program ATP-III were met, modified for prediabetes (fasting glucose 5.56 - 6.95 mmol/L) [27] with male central obesity defined as a waist circumference > 95 cm, as assessed in the Turkish Adult Risk Factor study [26]. In 2% of individuals exhibiting two MetS components and missing data on fasting triglycerides, the MetS status of a previous survey was adopted. Diabetes was diagnosed with the criteria of the American Diabetes Association [28], namely by self-report, plasma fasting glucose > 7mmol/L or with 2-h postprandial glucose > 11.1 mmol/L. HOMA was calculated as (insulin (mIU/L) ×glucose (mmol/L))/ 22.5. Nonfatal coronary heart disease (CHD) was identified by presence of angina pectoris, a history of myocardial infarction with/without accompanying Minnesota codes of the ECG [29] or a history of myocardial revascularization. Typical angina and, in women, age > 45 years, were prerequisites for a diagnosis when angina was isolated. ECG changes of “ischemic type” of greater than minor degree (Codes 1.1-2, 4.1-2, 5.1-2, 7.1) were considered as myocardial infarct sequelae or myocardial ischemia, respectively.
Data statistical analysis
This study is a cross-sectional population-based study. The study population characteristics according to gender are reported as mean values ± standard deviations for continuous variables and proportions for categorical variables. Graphical and statistical analyses were performed using GraphPad Prism and SPSS. Two sided t-tests and Fisher's exact test were used to analyze the differences between means and proportions of two groups, respectively. Spearman’s correlation coefficients served to analyze univariate correlations. A value of P < 0.05 was considered statistically significant.
| Results | ▴Top |
The study sample consisted of a random sampling of 224 adult Turkish men and women from the TARF study. The average age of the sample population was 51.5 yrs. This group was middle-aged to older, overweight (mean BMI = 28.1 men and 29.9 women), prone to abdominal obesity and similar to the overall cohort of the Turkish Adult Risk Factor study. In the group as a whole, presence of cardiometabolic disorders (at least one of these disorders; CHD, MetS, hypertension (HT), T2D) was 60.7% (60.8% in men, 60.6% in women). The study group characteristics and metabolic profiles, separated based on gender are shown in Table 1. There was no significant difference in age, waist and blood pressure values between men and women, but there were significant differences in height (men > women, P < 0.001) and BMI (women > men, P < 0.01). In general there was no significant difference between men and women in distribution of cardiovascular and metabolic disorders (Table 1). Total cholesterol, HDL-C, TG, glucose, creatinine, apolipoprotein A1, and (as expected) sex hormone binding globulin (SHBG), and testosterone were significantly different between men and women. However, apolipoprotein B and C-reactive protein, were not significantly different. The ASP values ranged from 51 to 1277 (nmol/L) in men with a geometric mean value of 305 nmol/L (25th percentile = 197.00, 75th percentile = 378.5) and 271 nmol/L (25th percentile = 192.8, 75th percentile = 484.3) in women (P = 0.059) (Table 1).
 Click to view | Table 1. Characteristics of the Study Population According to Sex |
In Figure 1, geometric mean ASP concentrations based on presence/absence of specific cardiometabolic disorders (CHD, metabolic syndrome, type-2 diabetes and hypertension) is shown in men and women. Levels were non-significantly higher in men with MetS and diabetes than without. Otherwise, including overall in women, ASP concentrations were non-significantly lower in the presence of cardiometabolic disorders.
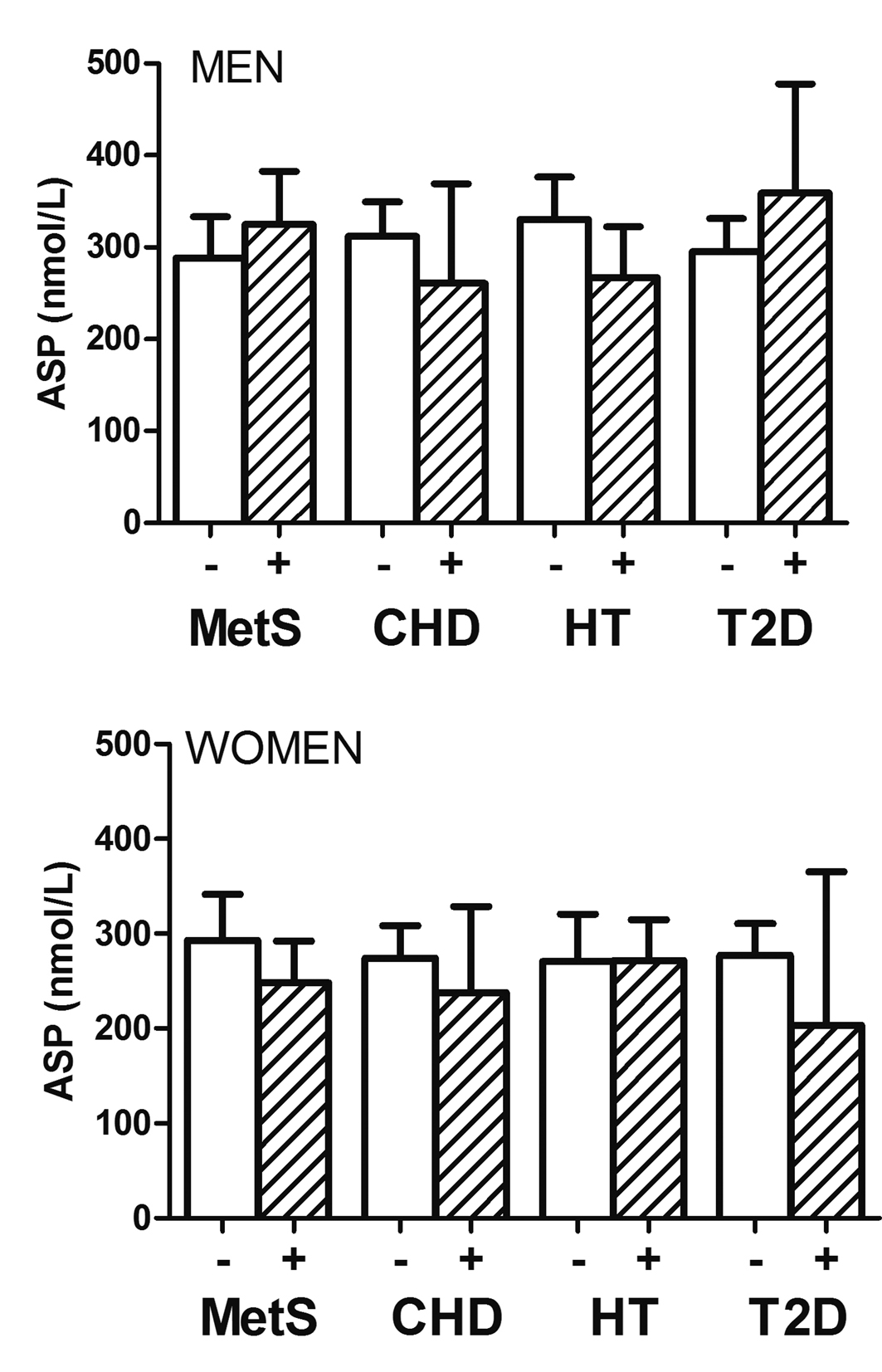 Click for large image | Figure 1. Geometric mean of plasma ASP concentrations in women (lower panel) and men (upper panel) without and with CHD, metabolic syndrome (MetS), Type-2 diabetes (T2D) and hypertension (HT). Levels were non-significantly higher in men with MetS and Type-2 diabetes than without. Otherwise, including overall in women, ASP concentrations were non-significantly lower in the presence of cardiometabolic disorders. Analysis used log-transformed ASP values. |
Correlation of ASP with various parameters in these subgroups is shown in Table 2. Overall, subjects with hypertension (HT) had several factors that correlated with ASP including SHBG, C-reactive protein (CRP), fibrinogen and creatinine. In subjects without hypertension there were positive correlations with height and negative with LDL-C. Similarly, ASP correlated with height, HDLC and SHBG in subjects with metabolic syndrome, but not in those without.
 Click to view | Table 2. Spearman Correlation Coefficients of ASP With Certain Variables According to Metabolic Disorders |
Correlation analysis of ASP was then performed stratified by sex, presence of MetS or other metabolic disorders (Table 3). Overall, correlations with ASP were stronger in men than in women in whom certain variables exhibited the opposite direction to those in men. Correlations of ASP overall in men were significant and positive with height, triglyceride, and glucose and tended to be so with CRP, with an inverse correlation with age and SHBG. In men with cardiometabolic disorders or with MetS, these correlations persisted and even tended to be accentuated as compared to men without MetS. Overall these correlations with ASP were in the opposite direction in women, even in healthy women. Significant inverse correlations were displayed with fasting glucose, LDL cholesterol, and testosterone and tended to be so with total cholesterol and fasting TG. Significant positive correlations with waist girth, (diastolic) BP, and age have been shown. These correlations were strongly attenuated in the presence of MetS or otherwise “non-healthy” women. Correlation of ASP to height tended to be inversely, although very weakly, correlated in “non-healthy” women.
 Click to view | Table 3. Spearman Correlation Coefficients of ASP With Certain Variables According to Gender |
| Discussion | ▴Top |
The salient findings in this population-based cross-sectional study among middle-aged Turkish adults were: 1) Higher average ASP concentrations in comparison with those reported in other ethnicities. 2) Diverging correlations across genders between ASP levels on the one hand and TG, glucose and height among other risk variables.
The average concentration of ASP in all Turkish subjects in this study was generally higher (median = 292 nmol/L) than in other studies [9]. Factors such as obesity, metabolic disorders, ethnic and nutritional differences may all contribute to this [9]. The ASP values measured here were higher than other ethnic groups such as Caucasian [12, 30, 31], African American [30], Pima Indian [12], Inuit from Nunavik [31] and Chinese [32] that have been measured so far, although the present group also contained a high percentage of known non-healthy subjects. ASP values were more similar to severely obese subjects from a North American cohort [11]. Further, there was a borderline sex difference (P = 0.059) between men and women, which was not seen in a normal-weight North American population, although there were differences between severely obese men and women [11]. As mentioned above, the present group has a high percentage of overweight and obese subjects as well as metabolic disorders (hypertension, metabolic syndrome, cardiovascular disease, type 2 diabetes), although this is a random representative sampling of the TARF study, and was not selected for the presence of metabolic disorders. Previous studies have shown that obese subjects, T2D subjects and subjects with cardiovascular diseases have higher ASP levels (58 - 400%) vs. normal weight controls [9]. A study by Yang et al. showed an increased ASP level in diabetic subjects even in the absence of obesity [15], and this was also true of women with polycystic ovary disease [18]. All of these factors likely contributed to the higher ASP levels.
The level of ASP may also be influenced by the concentration of its precursors [9]. ASP precursor components adipsin, C3, and factor B increase in obesity by approximately 30% for C3, 45% for factor B, and 37% for adipsin, however, small changes in substrate (C3) and enzyme (adipsin) may produce much larger changes in product (ASP) [33]. In several studies, C3 mRNA expression has been reported to be increased in older vs. younger subjects and in omental vs. subcutaneous tissue [9].
A number of studies have demonstrated that plasma ASP correlates positively with various indices of body size [9]. This includes BMI and percentage ideal body weight [17, 34], waist to thigh or waist to hip ratio [32], total fat mass or percentage body fat [17, 35]. In this study, BMI in non-healthy men had a significant positive correlation with ASP concentration and waist circumference, in women (without metabolic syndrome), this also correlated. Height was a body size marker that consistently correlated significantly with ASP in men, regardless of the group tested. Stepwise multiple forward analysis in this study indicated that height was an independent covariate of ASP only in men (results are not shown). In Caucasian population studies, it has been shown that height has an inverse association with CVD [36]. However, in rapidly developing populations, the protective effect of height on cardiovascular mortality or its risk factors is less obvious [37]. In an Asian (Hong Kong) population, height was inversely associated with increased blood pressure and raised fasting plasma glucose but only after adjustment for central obesity [37].
In both the present study and other studies, ASP consistently correlated with plasma lipid profile, factors commonly related to metabolic syndrome, diabetes and cardiovascular disease. Whether the increase in plasma ASP is a cause or a consequence of abnormal lipid metabolism cannot be determined simply by correlation studies, but examination of ASP function is supportive of linkages.
It has been proposed that a decrease in the ASP cell-surface receptor concentration or ASP response are related to hyperapoB, and that an elevated plasma ASP in patients with hyperapoB identifies individuals likely to have reduced cellular response to ASP stimulated peripheral TG synthesis and glucose transport [38]. Further, there are strong supports that ASP binding and subsequent response may be a significant factor in determining regional differences in fat distribution [39].
Enhanced ASP action in subcutaneous tissue can lead to an increase in triglyceride storage. Impaired ASP action in visceral adipose tissue, however, can contribute to decreased efficiency of triglyceride storage and increase circulating fatty acid fluxes, leading to metabolic imbalance and disorders that are commonly associated with visceral obesity, such as diabetes and cardiovascular disease [39]. Reduced adipose tissue response to ASP could contribute to an increased fatty acid flux, which leads to stimulation of hepatic apolipoprotein B lipoprotein production, explaining the associations between plasma ASP and lipid parameters [9]. With the identification of an ASP receptor, C5L2, in adipose tissue and other insulin-sensitive tissues [40] the presence of chronically elevated levels of ASP, in conjunction with insulin resistance and plasma lipid abnormalities, would be consistent with the hypothesis of 'ASP resistance' [14, 41].
Correlation between plasma ASP and other factors was different between men and women, and sex hormones could play a partial role in this. Overall, plasma ASP levels in normal weight girls were significantly higher than normal weight boys [32]. An in vitro study by Wen et al. [42] indicated that physiological concentrations of progesterone as well as high concentrations of testosterone induce ASP resistance in both adipocytes and preadipocytes, although other explanations are possible. While ASP may be increased as a response to insulin resistance, some cellular studies support the interpretation that the alteration in ASP pathway may be a direct response to the effect of the sex steroid hormones on downregulation of C5L2. For instance, physiological increases in sex steroid hormones in PCOS (polycystic ovary syndrome) and late pregnancy likely induce decreased C5L2 expression and signaling, leading to ASP resistance [18, 43].
A study by Xia and Cianflone [33] demonstrated marked changes in expression of factors involved in ASP generation associated with increased obesity that were very different in men and women. Specifically, with increased BMI, in women there was a decreased expression of C3 and adipsin, while in men there was increased expression of C3, factor B, and adipsin. In women, subcutaneous tissue was the primary target, while in men visceral tissue was more often affected. These results also demonstrated a greater relative expression in visceral than subcutaneous tissue with development of obesity in both men and women. Koistinen et al. demonstrated an increase in C3 mRNA in adipose tissue from obese men compared to lean men [41]. While these obese men only demonstrated a slight but not significant increase in plasma C3, they did have a substantial increase in plasma ASP. Interestingly, the level of C3 mRNA correlated inversely with glucose disposal rate but positively with BMI and postprandial triglyceride clearance [41].
Other divergent gender related associations have also been noted in this Turkish population. Gender influences the pro-inflammatory response to overall adiposity, central obesity and associated impaired function of HDL, with women affected to a greater extent [44]. Association of inflammatory mediators is largely independent of MetS components in men, but tends to act in conjunction in women [44]. Gender modulates response of cardiometabolic risk variables to moderate alcohol consumption and smoking status [44]. Aggregation of Lp(a) to apoA1 to form an immune complex contributes as a CHD risk factor [44] and gender divergence in Lp(a) and LP-PLA2 has also been noted in this population [45]. This inverse correlation, particularly in women, is in line with an enhanced role of pro-inflammatory status/oxidative stress in the pathogenesis of cardiovascular risk in Turkish women compared with men, and might relate to various HDL parameters (HDL, apoA1, apoCIII) [45]. The present findings pertaining to divergent associations in genders of fasting glucose, triglycerides and height (and circulating total testosterone) could be consistent with a notion that ASP is related to variations in Lp(a) and LP-PLA2 (data not shown) and possibly to HDL and its apolipoproteins, reflecting a balance in anti- and pro- inflammatory activities.
This population-based study has potential limitations in being cross-sectional in design wherein the causal role of ASP in cardiometabolic risk cannot be assessed. Secondly, the sample size for some cardiometabolic disorders (CHD, T2D) was less than adequate and precluded correlation analyses in subgroups. On the other hand, the availability of diverse lipid and non-lipid parameters, and identification of various cardiometabolic disorders in a population-based study comprising adults prone to enhanced low-grade inflammation, form its strength.
We conclude that ASP levels not only reflect dysglycemia and atherogenic dyslipidemia in men but also other inflammatory and anti-inflammatory biomarkers as well as height. The addition of MetS marginally accentuates these associations. In contrast, in women, correlations with ASP (notably triglycerides, glucose, LDL-C, SHBG and height) are in the opposite direction, as are those with serum Lp(a) (data not shown), in the absence of currently defined MetS.
Grant Support
The Turkish Adult Risk Factor surveys were supported by the Turkish Society of Cardiology and various pharmaceutical companies in Istanbul, Turkey, over the years (AO). KC is the recipient of a Canada Research Chair in Adipose Tissue and CIHR funding (64446).
Abbreviations
Apo: apolipoprotein; ASP: Acylation stimulating protein; BMI: Body mass index; CVD: Cardiovascular diseases; CHD: Coronary heart disease; HT: hypertension; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; MetS: Metabolic syndrome; SHGB: Sex hormone-binding globulin; T2D: Type 2 diabetes; TG: Triglyceride; TARF: The Turkish Adult Risk Factor study
| References | ▴Top |
- Hoole SP, Seddon MD, Poulter RS, Mancini GB, Wood DA, Saw J. Fame comes at a cost: a Canadian analysis of procedural costs in use of pressure wire to guide multivessel percutaneous coronary intervention. Can J Cardiol. 2011;27(2):262 e261-262.
pubmed - Camhi SM, Katzmarzyk PT. Prevalence of cardiometabolic risk factor clustering and body mass index in adolescents. J Pediatr. 2011;159(2):303-307.
pubmed doi - Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168(15):1617-1624.
pubmed doi - Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20(2):140-146.
pubmed doi - Gualillo O, Gonzalez-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends Cardiovasc Med. 2007;17(8):275-283.
pubmed doi - Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347-355.
pubmed doi - Armani A, Mammi C, Marzolla V, Calanchini M, Antelmi A, Rosano GM, Fabbri A, et al. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J Cell Biochem. 2010;110(3):564-572.
pubmed doi - Onat A, Can G, Rezvani R, Cianflone K. Complement C3 and cleavage products in cardiometabolic risk. Clin Chim Acta. 2011;412(13-14):1171-1179.
pubmed doi - Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta. 2003;1609(2):127-143.
pubmed doi - Maslowska M, Legakis H, Assadi F, Cianflone K. Targeting the signaling pathway of acylation stimulating protein. J Lipid Res. 2006;47(3):643-652.
pubmed doi - Maslowska M, Vu H, Phelis S, Sniderman AD, Rhode BM, Blank D, Cianflone K. Plasma acylation stimulating protein, adipsin and lipids in non-obese and obese populations. Eur J Clin Invest. 1999;29(8):679-686.
pubmed doi - Weyer C, Pratley RE. Fasting and postprandial plasma concentrations of acylation-stimulation protein (ASP) in lean and obese Pima Indians compared to Caucasians. Obes Res. 1999;7(5):444-452.
pubmed - Yesilova Z, Ozata M, Oktenli C, Bagci S, Ozcan A, Sanisoglu SY, Uygun A, et al. Increased acylation stimulating protein concentrations in nonalcoholic fatty liver disease are associated with insulin resistance. Am J Gastroenterol. 2005;100(4):842-849.
pubmed doi - Ozata M, Gungor D, Turan M, Ozisik G, Bingol N, Ozgurtas T, Ozdemir IC. Improved glycemic control increases fasting plasma acylation-stimulating protein and decreases leptin concentrations in type II diabetic subjects. J Clin Endocrinol Metab. 2001;86(8):3659-3664.
pubmed doi - Yang Y, Lu HL, Zhang J, Yu HY, Wang HW, Zhang MX, Cianflone K. Relationships among acylation stimulating protein, adiponectin and complement C3 in lean vs obese type 2 diabetes. Int J Obes (Lond). 2006;30(3):439-446.
pubmed - St-Pierre DH, Cianflone K, Smith J, Coderre L, Karelis AD, Imbeault P, Lavoie JM, et al. Change in plasma acylation stimulating protein during euglycaemic-hyperinsulinaemic clamp in overweight and obese postmenopausal women: a MONET study. Clin Endocrinol (Oxf). 2009;70(4):539-546.
pubmed doi - Cianflone K, Zhang XJ, Genest J, Jr., Sniderman A. Plasma acylation-stimulating protein in coronary artery disease. Arterioscler Thromb Vasc Biol. 1997;17(7):1239-1244.
pubmed - Wu Y, Zhang J, Wen Y, Wang H, Zhang M, Cianflone K. Increased acylation-stimulating protein, C-reactive protein, and lipid levels in young women with polycystic ovary syndrome. Fertil Steril. 2009;91(1):213-219.
pubmed doi - Tang JH, Wen Y, Wu F, Zhao XY, Zhang MX, Mi J, Cianflone K. Increased plasma acylation-stimulating protein in pediatric proteinuric renal disease. Pediatr Nephrol. 2008;23(6):959-964.
pubmed doi - Onat A, Hergenc G, Can G. Abdominal obesity criteria for Turkish men and women, and relevance of smoking for obesity/obesity and abdominal obesity; an alarming challenge for cardio-metabolic risk in Turkish adults. Anadolu Kardiyol Derg. 2009;9(2):147; author reply 147.
pubmed - Seidell JC. Prevalence and time trends of obesity in Europe. J Endocrinol Invest. 2002;25(10):816-822.
pubmed - Onat A. Risk factors and cardiovascular disease in Turkey. Atherosclerosis. 2001;156(1):1-10.
pubmed doi - Sanisoglu SY, Oktenli C, Hasimi A, Yokusoglu M, Ugurlu M. Prevalence of metabolic syndrome-related disorders in a large adult population in Turkey. BMC Public Health. 2006;6:92.
pubmed - Onat A, Hergenc G, Keles I, Dogan Y, Turkmen S, Sansoy V. Sex difference in development of diabetes and cardiovascular disease on the way from obesity and metabolic syndrome. Metabolism. 2005;54(6):800-808.
pubmed doi - Saleh J, Summers LK, Cianflone K, Fielding BA, Sniderman AD, Frayn KN. Coordinated release of acylation stimulating protein (ASP) and triacylglycerol clearance by human adipose tissue in vivo in the postprandial period. J Lipid Res. 1998;39(4):884-891.
pubmed - Onat A, Sari I, Hergenc G, Yazici M, Uyarel H, Can G, Sansoy V. Predictors of abdominal obesity and high susceptibility of cardiometabolic risk to its increments among Turkish women: a prospective population-based study. Metabolism. 2007;56(3):348-356.
pubmed doi - Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
pubmed doi - Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160-3167.
pubmed doi - Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. Geneva, World Health Organization, 2nd edn. 1982;p:124-127.
- Scantlebury-Manning T, Bower J, Cianflone K, Barakat H. Racial difference in Acylation Stimulating Protein (ASP) correlates to triglyceride in non-obese and obese African American and Caucasian women. Nutr Metab (Lond). 2009;6:18.
pubmed - Smith JD, Cianflone K, Dewailly E, Chateau-Degat ML, Vohl MC, Julien P. Acylation stimulating protein is higher in Inuit from Nunavik compared to a southern Quebec population. Int J Circumpolar Health. 2009;68(5):421-432.
pubmed - Wamba PC, Mi J, Zhao XY, Zhang MX, Wen Y, Cheng H, Hou DQ, et al. Acylation stimulating protein but not complement C3 associates with metabolic syndrome components in Chinese children and adolescents. Eur J Endocrinol. 2008;159(6):781-790.
pubmed doi - Xia Z, Cianflone K. Acylation-stimulating protein precursor proteins in adipose tissue in human obesity. Metabolism. 2003;52(10):1360-1366.
pubmed doi - Cianflone K, Lu H, Smith J, Yu W, Wang H. Adiponectin, acylation stimulating protein and complement C3 are altered in obesity in very young children. Clin Endocrinol (Oxf). 2005;62(5):567-572.
pubmed doi - de Lind van Wijngaarden RF, Cianflone K, Gao Y, Leunissen RW, Hokken-Koelega AC. Cardiovascular and metabolic risk profile and acylation-stimulating protein levels in children with Prader-Willi syndrome and effects of growth hormone treatment. J Clin Endocrinol Metab. 2010;95(4):1758-1766.
pubmed doi - McCarron P, Okasha M, McEwen J, Smith GD. McCarron et al. respond to "height-cardiovascular disease relation": are all risk factors equal? Am J Epidemiol. 2002;155(8):690-691.
pubmed - Schooling CM, Thomas GN, Leung GM, Ho SY, Janus ED, Lam TH. Is height associated with cardiovascular risk in Chinese adults? Epidemiology. 2007;18(2):274-278.
pubmed doi - Zhang XJ, Cianflone K, Genest J, Sniderman AD. Plasma acylation stimulating protein (ASP) as a predictor of impaired cellular biological response to ASP in patients with hyperapoB. Eur J Clin Invest. 1998;28(9):730-739.
pubmed doi - Saleh J, Christou N, Cianflone K. Regional specificity of ASP binding in human adipose tissue. Am J Physiol. 1999;276(5 Pt 1):E815-821.
pubmed - Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J Biol Chem. 2003;278(13):11123-11129.
pubmed doi - Koistinen HA, Vidal H, Karonen SL, Dusserre E, Vallier P, Koivisto VA, Ebeling P. Plasma acylation stimulating protein concentration and subcutaneous adipose tissue C3 mRNA expression in nondiabetic and type 2 diabetic men. Arterioscler Thromb Vasc Biol. 2001;21(6):1034-1039.
pubmed doi - Wen Y, Wang H, MacLaren R, Lu H, Hu XF, Cianflone K. Sex steroid hormones induce acylation stimulating protein resistance in 3T3-L1 adipocytes. J Cell Biochem. 2008;105(2):404-413.
pubmed doi - Saleh J, Cianflone K, Chaudhary T, Al-Riyami H, Al-Abri AR, Bayoumi R. Increased plasma acylation-stimulating protein correlates with hyperlipidemia at late gestation. Obesity (Silver Spring). 2007;15(3):646-652.
pubmed doi - Onat A, Hergenc G. Low-grade inflammation, and dysfunction of high-density lipoprotein and its apolipoproteins as a major driver of cardiometabolic risk. Metabolism. 2011;60(4):499-512.
pubmed doi - Onat A, Hergenc G, Can G, Ugur M, Nartop F. Dual activity of serum lipoprotein-associated phospholipase A(2) yielding positive and inverse associations with cardiometabolic risk. Clin Chem Lab Med. 2011;49(8):1349-1357.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.









