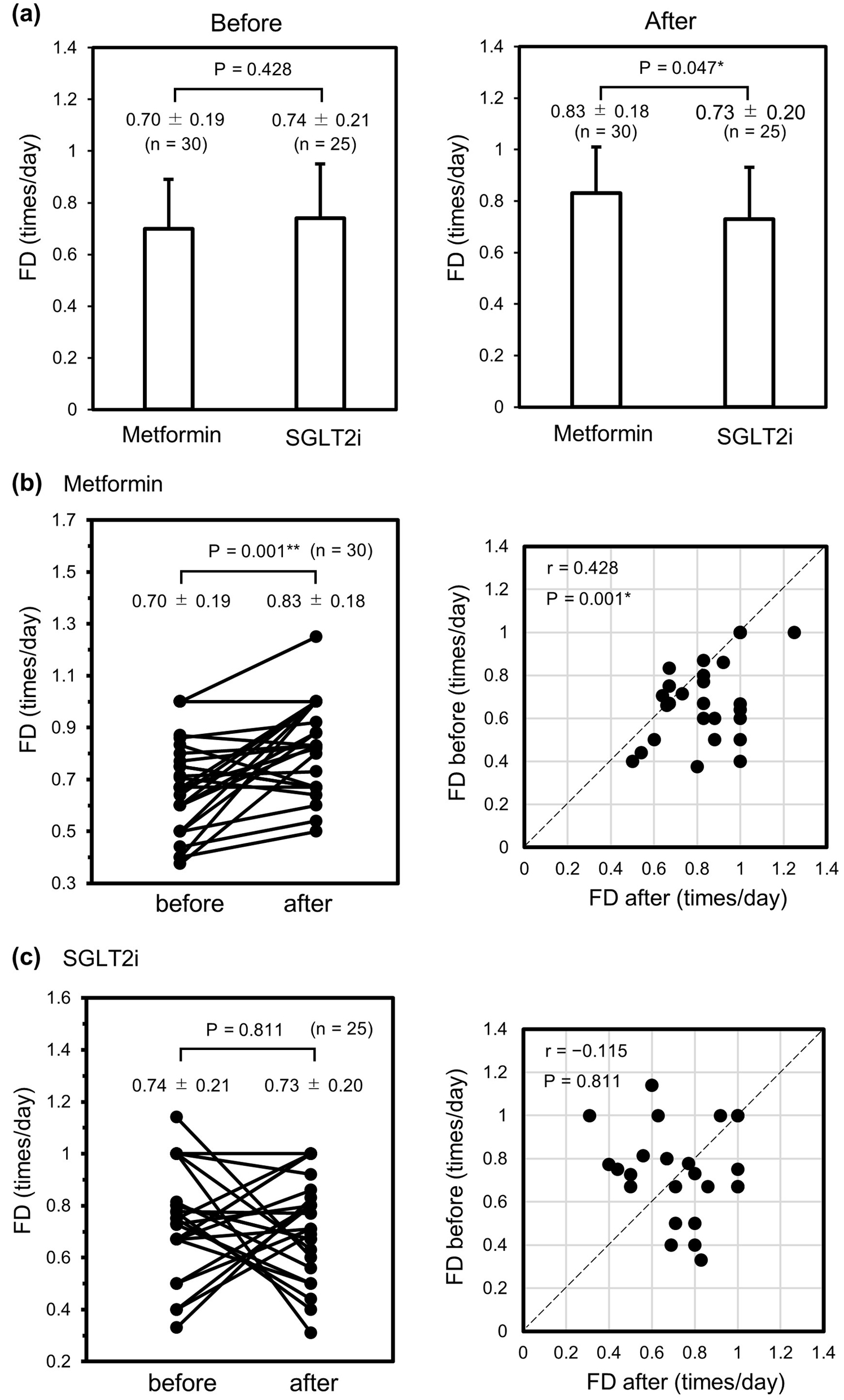
Figure 1. Changes in FD before and after administration of metformin or an SGLT2i during hospitalization. (a) Comparison of FD before (left panel) and after (right panel) initiation of metformin (n = 30) or an SGLT2i (n = 25). (b) Line (left) and dot plot (right) comparisons of FD among individuals in the metformin group. (c) Line (left) and dot plot (right) comparisons of FD among individuals in the SGLT2i group. Data were analyzed using the unpaired t-test in (a) and the paired t-test in (b) and (c). *P < 0.05. **P < 0.01. FD: frequency of defecation; SGLT2i: sodium-glucose cotransporter-2 inhibitor.
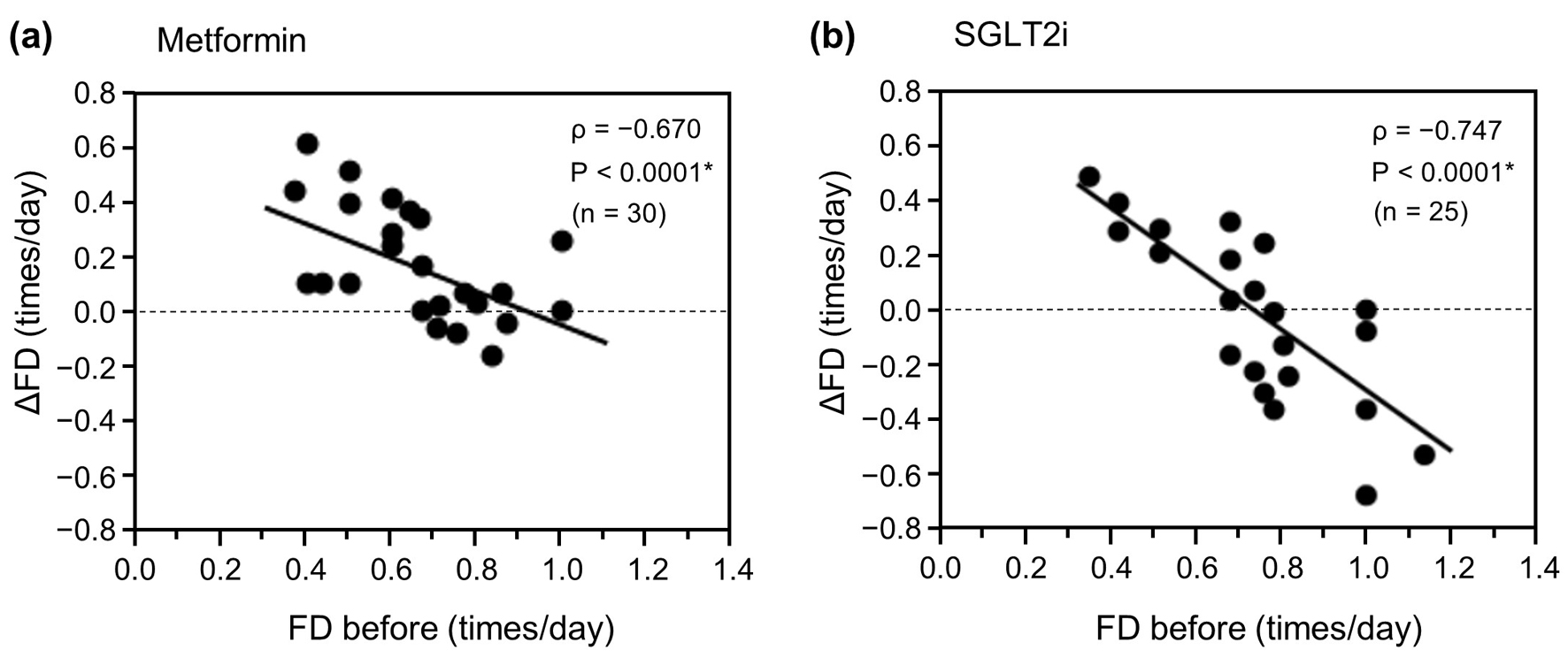
Figure 2. Relationship between ΔFD and FD before initiation of metformin or an SGLT2i. (a) Metformin group (n = 30). (b) SGLT2i group (n = 25). Data were analyzed using Spearman’s correlation analysis. *P < 0.01. ΔFD: change in FD; FD: frequency of defecation; SGLT2i: sodium-glucose cotransporter-2 inhibitor.
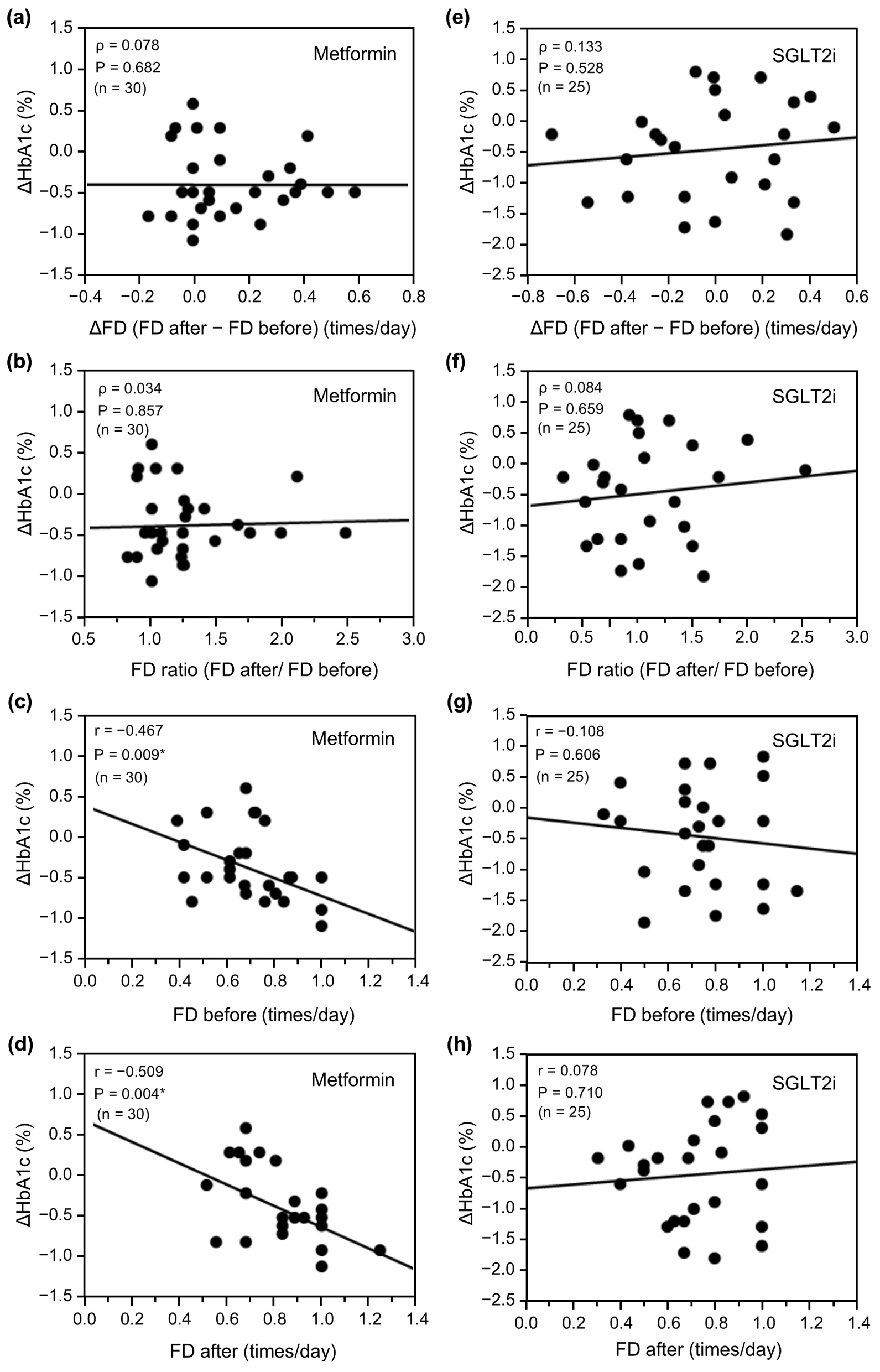
Figure 3. Relationship between ΔHbA1c and change in FD (ΔFD or FD ratio) or FD before and after initiation of metformin (n = 30) or an SGLT2i (n = 25). (a) ΔFD after initiation of metformin, (b) FD ratio before and after initiation of metformin, (c) FD before initiation of metformin, (d) FD after initiation of metformin, (e) ΔFD after initiation of SGLT2i, (f) FD ratio before and after initiation of SGLT2i, (g) FD before initiation of SGLT2i, and (h) FD after initiation of SGLT2i. Data in (a), (b), (e), and (f) were analyzed using Spearman’s correlation analysis and data in (c), (d), (g), and (h) were analyzed using the unpaired t-test. *P < 0.01. ΔHbA1c: change in glycated hemoglobin; ΔFD: change in FD; FD: frequency of defecation; SGLT2i: sodium-glucose cotransporter-2 inhibitor.


