| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 13, Number 2, June 2023, pages 70-74
Intensive Management of Poorly Controlled Type 2 Diabetes Using a Multidisciplinary Approach and Continuous Glucose Monitoring
Andrew J. Behnkea, c, David Woodfieldb
aSection of Endocrinology, Carilion Clinic, Roanoke, VA 24016, USA
bSection of Internal Medicine, Carilion Clinic, Roanoke, VA 24016, USA
cCorresponding Author: Andrew J. Behnke, Section of Endocrinology, Carilion Clinic, Roanoke, VA 24016, USA
Manuscript submitted October 14, 2022, accepted October 29, 2022, published online June 30, 2023
Short title: Management of T2D Using CGM
doi: https://doi.org/10.14740/jem844
| Abstract | ▴Top |
Background: The purpose of this study was to examine the effects of a weekly monitoring interaction using continuous glucose monitoring (CGM) in a population of poorly controlled type 2 diabetes patients.
Methods: This study was conducted in the outpatient clinical setting and examined levels of hemoglobin A1c (HbA1c) and time in range (TIR) glucose levels for 16 patients with poorly controlled type 2 diabetes as indicated by an HbA1c level of greater than 10%. The intervention included use of a continuous glucose monitor and weekly interactions either virtually or by telephone by one of the team members.
Results: After a 3-month period, HbA1c levels reduced from 11.79% to 7.88% (P < 0.01) with 100% of the subjects achieving HbA1c of less than 10%. There were no significant changes in the amount of additional diabetes medication or insulin dose.
Conclusions: The combination of CGM and frequent interaction in a brief (3 months) time frame may be a significant tool to improve glucose control in this high-risk population.
Keywords: Diabetes type 2; Continuous glucose monitoring; Team approach; Poor control
| Introduction | ▴Top |
Diabetes mellitus is a major cause of morbidity and mortality in the United States and the world. Aside from the difficulties with hyperglycemia, it is a significant comorbidity for many cardiovascular diseases [1]. A subgroup of patients with type 2 diabetes have poorly controlled glucose levels. Data from the Centers for Disease Control estimate that 16% of all patients with type 2 diabetes have an A1c of > 9% (75 mmol/mol) [2]. Poor glucose control contributes to the increase in hospitalization and morbidity [3].
Intensive programs that address these issues have been used in several health care systems with proven benefit [4, 5]. These programs are sometimes referred to as diabetes boot camps and are modeled after the American Diabetes Association’s Standards of Care [1, 4, 5]. Interdisciplinary team management has been recommended by the Global Partnership for Effective Diabetes Management International Task Force on Diabetes Care [6]. Effective diabetes care teams should include a range of providers across clinic and community settings that provide the diabetes self-management education and support required for adequate disease management [7].
Additionally, continuous glucose monitoring (CGM) has become a standard for glucose monitoring. Results of recent studies have shown that adding a CGM device resulted in improved glucose control by as much as 1.5% reduction in hemoglobin A1c (HbA1c) levels after 3 months [8]. Other studies have demonstrated that use of CGM reduces hospitalization and medical costs [9]. When intermittent glucose monitoring has been combined with a multidisciplinary diabetes management program, the effect on HbA1c reduction has been shown to be as much as 3.1% [10]. We are not aware of studies combining CGM with intensive programs in patients with poorly controlled type 2 diabetes.
The goal of this project was to assess the benefit of an intensive diabetes education program and CGM to reduce the level of glucose in patients with poorly controlled type 2 diabetes.
| Materials and Methods | ▴Top |
Setting
Carilion Clinic Endocrinology is an outpatient endocrinology practice that is a subset of Carilion Clinic. It is associated with Virginia Polytechnic Institute and State University, a large academic medical center in southwest Virginia. The Endocrinology Clinic is staffed by six physicians. Additionally, as a part of the partnership with the Virginia Tech School of Medicine and associated Internal Medicine and Family Medicine residencies, resident physicians routinely work under the supervision of attending physicians.
Participants
Adults with uncontrolled type 2 diabetes who were patients in the Carilion Clinic Endocrinology practice were identified. Inclusion criteria included age 18 or older, HbA1c level of 9% (75 mmol/mol) or greater, and willingness to participate in the multidisciplinary program.
Intervention
A 3-month interdisciplinary program was developed to bring together multiple specialists. Components of this program included the following: referral to Diabetes Self-Management Education (DSME) with the goal of completing two visits during the program, referral to clinical pharmacist, use of CGM device, and weekly check-in with a physician at the endocrinology clinic.
Weekly follow-up visits fell into one of two categories. If a patient was seen in-person at the clinic, that visit would serve as their weekly follow-up. Otherwise, follow-ups were conducted virtually through a secure patient messaging portal or over the phone. These visits consisted of reviewing CGM data virtually (data were shared through either the Abbott Freestyle LibreView platform or Dexcom Clarity platform) when available. In cases where CGM data were unavailable virtually, patients were advised to either bring their CGM or glucometer into the office for download, or to enter blood glucose data into the messaging portal. Glucose data were then reviewed by a physician to assess the degree of glucose control and adjust the patient’s anti-hyperglycemic regimen as needed.
Patients were also referred to a clinical pharmacist, with a goal of at least one interaction. The role of an encounter with a clinical pharmacist was to assess for medication compliance, assist with difficulties obtaining medications (such as connecting patients with medication assistance programs or coupon cards in case of financial difficulty), and make recommendations regarding pharmacotherapy.
A referral was placed to the DSME program, where patients would meet with a certified diabetes care and education specialist to discuss a wide range of topics such as diet, exercise, medication adherence, and more based on individual patient need and preference. The goal was for both an initial and follow-up encounter with DSME to be completed within the 3-month program.
Patients were prescribed one of two CGM devices, the Abbott Freestyle Libre 2 or the Dexcom G6. Device selection was based on patient preference. When available, an initial sensor kit was provided to the patient in the office and future refills were prescribed. Regardless of the device, patients were instructed on how to connect the CGM with the respective online platform and share their glucose data with the practice.
Outcomes
The primary outcome was change in HbA1c between the start and completion of the program. Secondary outcomes included change in time in range (TIR) as defined by percentage of time wearing CGM where blood glucose level was between 3.9 and 10 mmol/L (70 and 180 mg/dL), change in average blood glucose level, change in body mass index (BMI), change in total daily dose (TDD) of insulin, and change in number of non-insulin anti-hyperglycemic medications.
Data collection
The initial HbA1c was obtained at time of enrollment during the first visit with an endocrinologist. If the patient already had an HbA1c drawn and available within the preceding 3 months, this value was used for the starting HbA1c instead. The final HbA1c was collected at the patient’s last appointment. If the patient was unable to attend the final 3-month appointment, the closest prior HbA1c was used, whether that was performed through the endocrinology office or with the patient’s primary care provider.
TIR was collected based on the first available 2-week period of data following initiation of CGM. These data were collected using the online CGM platform reports. For patients who were already using CGM prior to initiation of the program, the 2 weeks immediately preceding the first visit were used to calculate initial TIR. Final TIR was calculated based on the 2 weeks immediately preceding the final visit or follow-up. Average blood glucose data were available on the same CGM reports and were calculated using the same intervals as TIR. Average glucose and TIR were omitted if patients did not utilize CGM.
Statistical analysis
Data were summarized using means and standard deviation (SD) for continuous variables and percentages for categorical variables. Data points including HbA1c, TIR, average glucose level, BMI, TDD, and number of non-insulin anti-hyperglycemic medications were assessed for normal distribution with skewness and kurtosis. If both fell within the range of -2 to +2, a two-tailed paired t-test was performed and an associated P-value calculated to assess statistical significance. We used an alpha level of 0.05 for all statistical tests.
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki declaration. The Carilion Clinic Institutional Review Board (IRB) has determined that the project does not meet the definition of human subjects research as outlined in 45 CFR 46.102(d), and therefore does not require IRB oversight or approval.
| Results | ▴Top |
Demographics and characteristics
The completion of the intervention phase was completed as planned in July 2022. Twenty-one patients were identified from December 2021 through May 2022 who met the study criteria. Of these 21 patients, throughout the 3-month program, five subsequently discontinued prior to completion of the program. Data collected on patients who did not complete the program were excluded from analysis. Sixteen patients successfully completed the program. The mean age was 56.9 (SD 10.8) and the participants were predominantly female at 62.5% (Table 1). The mean initial HbA1c was 11.8% (105 mmol/mol).
 Click to view | Table 1. Baseline Characteristics |
Not all the prescribed interventions were successfully completed. For example, only 75% (n = 12) of study participants attended at least one of the two planned DSME appointments and only 38% (n = 6) attended both appointments. CGM usage was increased, with 81% (n = 13) of participants using CGM. Of patients completing the study with CGM usage, 100% (n = 13) utilized the Abbott Freestyle Libre 2 system. Two participants who self-discontinued from the study utilized the Dexcom G6 system. Lastly, 81% (n = 13) of participants attended the virtual appointment with a clinical pharmacist.
The mean change in HbA1c (M = -3.91, SD = 2.09, N = 16) was significantly less than zero, t(15) = 7.49, two-tail P ≤ 0.001 (Fig. 1). For secondary outcomes, however, there were no statistically significant changes (Table 2). The mean change of TIR (M = 2.77, SD = 16.64, N = 13) was not statistically significantly increased, t(12) = -0.60, two-tail P = 0.56. The mean change of BMI (M = -0.55, SD = 1.53, N = 16) was not statistically significantly decreased either, t(15) = 1.43, two-tail P = 0.17.
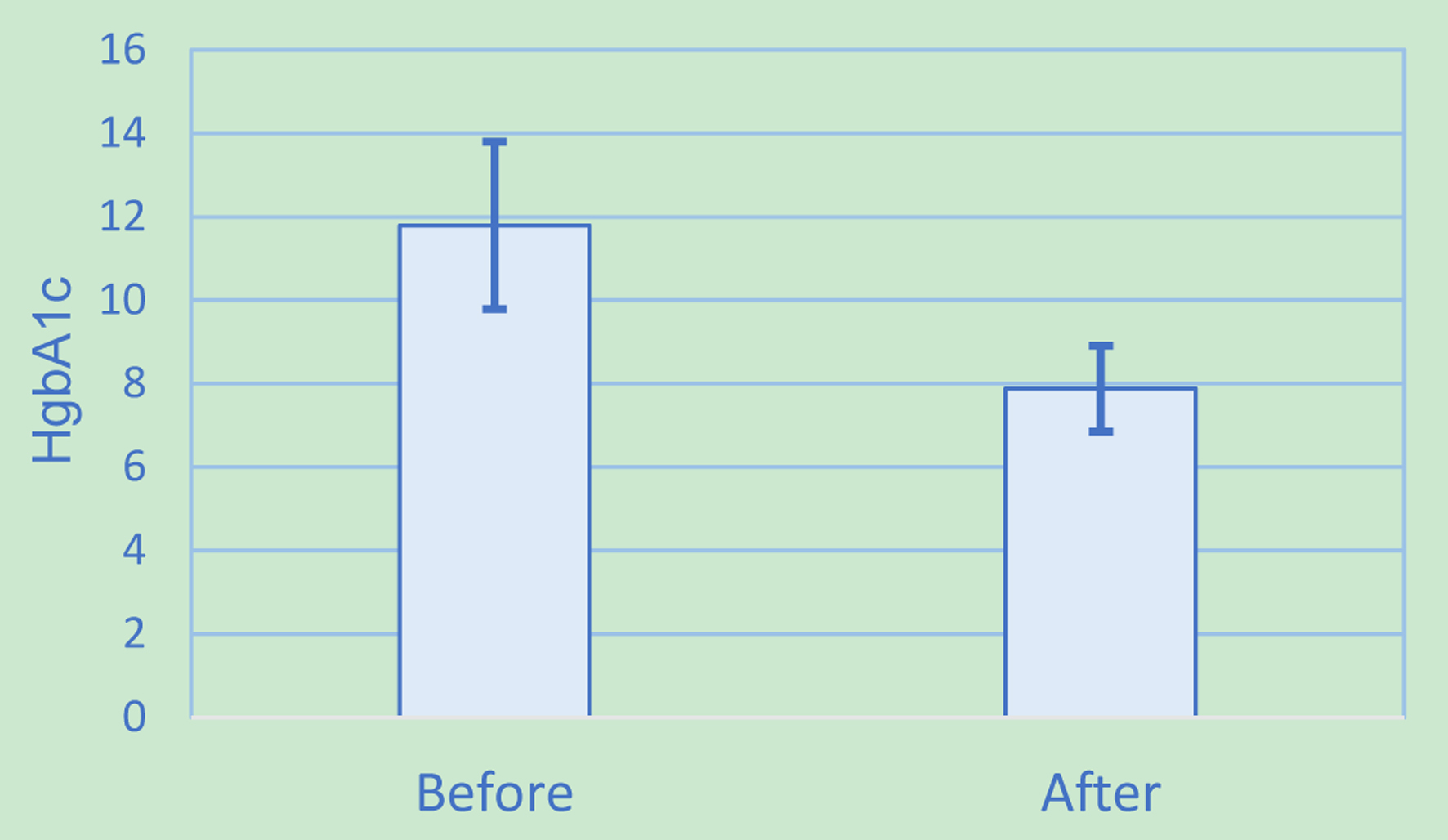 Click for large image | Figure 1. Hemoglobin A1c results. |
 Click to view | Table 2. Primary and Secondary Outcome Results |
| Discussion | ▴Top |
Control of type 2 diabetes in patients with HbA1c levels greater than 10% can be difficult to obtain. In this study, the combination of CGM, nutritional consultation, clinical pharmacy intervention, and frequent follow-up was able to significantly decrease glucose levels in a relatively brief period of time. We think that these combination factors might be synergistic and are eager to sort out which of them played a more prestigious role. The success was achieved without significantly increasing weight, insulin dose, or the number of non-insulin anti-hyperglycemic medications.
This study was a real-world scenario and was done without third-party financial support. It mimics the efforts that can be made in a clinic without having to provide significant resources. There were a few limitations identified in this study. Primarily, the limited number of patients enrolled in the study makes generalizing the results challenging. The resource-intensive nature of the weekly follow-ups made enrollment of greater numbers of patients challenging.
Several aspects of CGM usage posed limitations as well. This included the patients’ ability to obtain CGM devices. Additionally, for those patients who did begin CGM, initiation of timing following initial visit was variable. This limits the utility of the initial TIR and average blood glucose. Selection bias may have also been a factor in the difference between the reduction in HbA1c verses the less significant reduction in TIR. Patients were referred to the Endocrinology Clinic and may have already adopted changes in treatment adherence and lifestyle modification before beginning the CGM use. The TIR was increased but the SD was large and the sample size was small resulting in a value that did not reach statistical significance. Additional studies are needed to further explore if this measurement is indeed increased in the study group.
Despite these limitations, the impact of an intensive multidisciplinary diabetes education program in combination with CGM usage was clear with a statistically significant decrease in HbA1c from 11.79% to 7.88% (105 to 63 mmol/mol). Although clear interventions through referrals to diabetic education and clinical pharmacy, CGM, and weekly follow-up were in place, consideration was also given to the impact of pharmacological therapy added or adjusted throughout the course of the study. To that end, other data points such as TDD of insulin and number of non-insulin hyperglycemic agents were also evaluated. TDD did minimally increase by 6.3 units; however, this increase was not significantly significant. Similarly, the number of non-insulin anti-hyperglycemic agents did not increase by a statistically significant amount. These findings suggest that pharmacological therapy adjustments played a minimal role in the change in HbA1c, as compared to the multidisciplinary program.
Acknowledgments
None to declare.
Financial Disclosure
No funding sources were used.
Conflict of Interest
No conflict of interest exists.
Informed Consent
The study was deemed not human research by the institutional IRB. In light of that no informed consent was obtained.
Author Contributions
Dr. Behnke designed the study, submitted the IRB documents and performed a majority of the literature review; recruited patients from the endocrinology clinic; assisted in the study implementation and monitoring; located the journal to report the study to and assisted in manuscript preparation. Dr. Woodfield reviewed the study protocol and assisted in the management of the study; analyzed the data and wrote the manuscript portion of the results and methods; participated in recruitment and monitoring of the study.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- American Diabetes Association. 1. Improving care and promoting health in populations: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S7-S14.
doi pubmed - Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
- Hartz A, Kent S, James P, Xu Y, Kelly M, Daly J. Factors that influence improvement for patients with poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2006;74(3):227-232.
doi pubmed - Reddy M, O’Donnell T, Murphy R, May KE, Reddy S. Diabetes boot camp: An intensive, team-based approach to glucose control and patient empowerment in type 2 diabetes. Diabetes. 2017;66(Supplement 1):A361.
- Deichmann RE, Cazabon P, Asher T, Cripe B, Griffin R, Dornelles A, Denton GD. Long-term effects of a diabetes boot cAMP on measures of diabetic care. Ochsner J. 2015;15(1):13-18.
- McGill M, Blonde L, Chan JCN, Khunti K, Lavalle FJ, Bailey CJ, Global Partnership for Effective Diabetes M. The interdisciplinary team in type 2 diabetes management: Challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21-27.
doi pubmed - Schmittdiel JA, Gopalan A, Lin MW, Banerjee S, Chau CV, Adams AS. Population health management for diabetes: health care system-level approaches for improving quality and addressing disparities. Curr Diab Rep. 2017;17(5):31.
doi pubmed - Noar A, Welsh J, Price DA. 870-P: A1C reductions and improved patient-reported outcomes following CGM initiation in insulin-managed T2D. Diabetes. 2020;69(Supplement 1):870–P.
doi - Deshmukh H, Wilmot EG, Gregory R, Barnes D, Narendran P, Saunders S, Furlong N, et al. Effect of flash glucose monitoring on glycemic control, hypoglycemia, diabetes-related distress, and resource utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care. 2020;43(9):2153-2160.
doi pubmed - Magee MF, Baker KM, Fernandez SJ, Huang CC, Mete M, Montero AR, Nassar CM, et al. Redesigning ambulatory care management for uncontrolled type 2 diabetes: a prospective cohort study of the impact of a Boot Camp model on outcomes. BMJ Open Diabetes Res Care. 2019;7(1):e000731.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.









