| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 13, Number 1, February 2023, pages 20-32
Predictors of Perioperative Hypertensive Crisis in Patients With Pheochromocytoma: A Retrospective Study
Mozhgan Karimifara, d, Sina Abbaspoura , Awat Feizib, Mitra Heidarpourc
aIsfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
bDepartment of Biostatistics and Epidemiology, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran
cDepartment of Pathology, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
dCorresponding Author: Sina Abbaspour, Department of Internal Medicine, School of Medicine, Alzahra Hospital, Isfahan University of Medical Sciences, Isfahan 81746-73461, Iran
Manuscript submitted November 23, 2022, accepted December 7, 2022, published online February 25, 2023
Short title: Hypertensive Crisis in Pheochromocytoma
doi: https://doi.org/10.14740/jem853
| Abstract | ▴Top |
Background: Pheochromocytoma is a rare adrenal gland tumor. The definitive treatment is an adrenalectomy. Because of its secretory nature, appropriate preoperative treatment is essential to prevent hypertensive crisis (HTC) during surgery. Despite this management, HTC is common and can cause life-threatening complications. We aimed to study variables that may affect HTC despite preoperative management.
Methods: In a retrospective study, among 126 medical records of patients with adrenal tumors who were referred to Alzahra Hospital, Isfahan, Iran, between 2013 and 2021, 52 patients who took proper preparation for surgery were included.
Results: Analysis of these patients (aged 15 - 72 years, 30 females) showed that 12 patients (23.1%) experienced HTC. The mean age in the HTC group was 44.0 ± 15.3 and in the non-HTC group was 45.6 ± 13.2 (P = 0.724). Among many potential predictors, we observed in a multivariate analysis that patients with tumors size > 33.5 mm were at higher risk for experiencing HTC (P = 0.038, odds ratio (OR): 13.1, confidence interval (CI): 1.26 - 135.26); taking amlodipine to help reduce blood pressures (BPs) was another significant predictor (P = 0.05, OR: 5.1, CI: 0.97 - 56.74). Mean values of systolic BP (SBP) and diastolic BP (DBP) before surgery in the HTC group were more, although it was not statistically significant. Patients’ past medical history, 24-h urine metanephrine, normetanephrine, epinephrine, norepinephrine, vanillymandelic acid (VMA), and surgical technique were not significantly distributed between HTC and non-HTC patients (P > 0.05).
Conclusion: Tumor sizes > 33.5 mm and the necessity of administering amlodipine to control BP were predictors of HTC. Due to the rarity of pheochromocytoma, multicenter studies with larger sample sizes for providing more reliable results are suggested.
Keywords: Pheochromocytoma; Tumor size; Blood pressure; Phenoxybenzamine; Hypertensive crisis; Amlodipine; Hematology
| Introduction | ▴Top |
Pheochromocytoma, a rare catecholamine-producing neuroendocrine tumor (with an incidence of 0.8 per 100,000 persons per year in the general population) which originates from the medulla part of the adrenal gland, is associated with high morbidity and mortality when left untreated. It can either be unilateral or bilateral [1-4]. Symptoms include cardiovascular complications such as hypertension, palpitation, hyperglycemia, decreased intravascular volume [5, 6], headaches, diaphoresis and in more severe cases, a life-threatening crisis that manifests itself as encephalopathy (caused by high blood pressure (BP)), symptoms of neurological deficits and loss of consciousness, metabolic acidosis, and eventually death [7-9].
The approach to diagnosis and surgical treatment is well known, but preoperative management is still diverse [10]. Complete surgical excision is the definitive treatment of pheochromocytoma [11]. Because of its secretory nature, preoperative treatment of patients with pheochromocytoma is essential to prevent perioperative hemodynamic instability [12, 13]. During the surgery, catecholamines may be released by the tumor and cause hypertensive crisis (HTC), cardiac arrhythmias, cerebrovascular accidents, myocardial infarction or ischemia, pulmonary edema, and organ failure. Therefore, preparation before surgery is very important to reduce the risk of complications too [14-17].
Recommendations suggest pretreatment with an α-adrenergic receptor blocker to minimize the vasoconstrictive effects of catecholamines [8, 18]. Phenoxybenzamine (PhB) was approved by the US Food and Drug Administration in 1953 to be used in HTC, especially in patients with high levels of epinephrine and norepinephrine-secreting tumors, including pheochromocytoma. It binds covalently to α-adrenergic receptors, which inhibits these receptors in a non-competitive, long-term manner [19]. This drug is the preferred and the first-choice drug used to prepare patients before adrenalectomy in many medical centers (in comparison to selective α-1-adrenergic blocking agents (e.g., prazosin, terazosin, or doxazosin)). After the adequate α-adrenergic blockade, a β-adrenergic blockade is initiated usually 2 - 3 days before surgery. The β-adrenergic blocker should never be started first because blockade of peripheral β-adrenergic receptors without α-adrenergic receptor block can cause a further elevation in BP. Calcium channel blockers are the second drug in managing these patients [10, 20, 21]. On the second or third day of the α-adrenergic blockade, patients are encouraged to start a diet of high sodium content (> 5,000 mg daily) because of the catecholamine-induced volume contraction and the orthostasis associated with α-adrenergic blockade [21].
Patients are prepared for the surgery when BP is < 120/80 mm Hg (seated), with systolic blood pressure (SBP) > 90 mm Hg (standing) [21, 22]. There are differing opinions about the required PhB dose before surgery. Some references suggest that with sitting BP < 130/80 mm Hg and standing SBP > 90 mm Hg and a heart rate range of 60 - 80 beats/min, the patient is ready for adrenalectomy [18]. Some references suggest BP < 160/90 mm Hg, recommending 0.5 to 4 mg/kg dosing of PhB [23].
Despite proper preoperative preparation, HTC is unpredictable and common during pheochromocytoma resection. Recent studies have shown predictive values for HTC during surgery but the results are conflicting. They have shown that higher catecholamine levels, large tumors, α-blocker type, hydration status, and procedure type (open vs. laparoscopic) may be associated with this condition [4, 22, 24-26]. HTC is a stressful event during surgery because of its probable side effects mentioned earlier. Therefore, this study examines our center’s experience with pheochromocytoma resection in an attempt to identify patient/tumor-related factors predictive of HTC during surgery.
| Materials and Methods | ▴Top |
Patients and setting
After institutional ethics approval was obtained (ethical code: IR.MUI.MED.REC.1399.477, scientific code: 399417), a retrospective single-center study was done. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Data were reviewed from patients who were suspected of pheochromocytoma between March 2013 and October 2021, in the Alzahra Hospital affiliated with Isfahan University of Medical Sciences, Isfahan, Iran. The diagnosis was established by an increase in 24-h urine catecholamines more than two times of upper limit of the normal range or metanephrine > 900 µg or 24-h urine metanephrine more than 400 µg/day and localizing the tumor in imaging data [23, 27]. Patients with pheochromocytoma who underwent adrenalectomy with appropriate preparation for the surgery were included. As the main treatment is done with α-blockers, we included cases who were prepared with PhB for this procedure.
Among 126 medical records of patients with adrenal tumors, 74 patients’ records were excluded from this study because of indeterminate dose of PhB (two cases), no PhB being administered (14 cases, three of whom were suspected of pheochromocytoma and 11 were diagnosed with another disease), no adrenalectomy being done (eight cases), pheochromocytoma being ruled out (27 cases) and having missing data (23 cases). Important data which are worth mentioning include four cases diagnosed as aldosteronoma, 21 cases with Cushing syndrome, and two cases with myelolipoma. We also found three cases with neurofibromatosis and three cases with multiple endocrine neoplasia type 2 (MEN2). Finally, 52 patients who were diagnosed with pheochromocytoma (based on catecholamine levels, imaging, and clinical presentation) and underwent surgery with PhB preparation were included in the study.
Preoperative management of patients
We found out that patients diagnosed or strongly suspected of pheochromocytoma received pharmacological preparation for more than 10 days in most cases. All 52 patients received PhB, as the principal drug for preoperative BP control. Some patients received prazosin too (a competitive α-adrenergic receptor blocker). A β-adrenergic receptor antagonist (metoprolol or propranolol) was also prescribed for most patients 2 - 3 days before surgery. Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) and amlodipine (a calcium channel blocker) were added for more hemodynamic control, if necessary. A high-salt diet and increasing daily fluid intake were administered to prevent hypovolemia (on the second or third day of the α-adrenergic blockade). Patients were prepared for the surgery when BP was less than 120/80 mm Hg (seated), with SBP greater than 90 mm Hg (standing); both targets were modified based on age and comorbid diseases [21, 22].
Data collection
All data were obtained from either electronic or paper medical records. Basic demographic and clinical characteristics of patients included age, gender, past medical history, and pheochromocytoma-associated conditions (diabetes mellitus, hypertension, hypothyroidism, neurofibromatosis, MEN2, ischemic heart disease and previous history of pheochromocytoma).
Preoperative data included: clinical presentation recorded in patients’ medical records (palpitation, headache, increased BP, dyspnea, and abdominal pain) and the duration of these presentations, tumor size and localization (size, left or right or both-sided which were evaluated by abdominal magnetic resonance imaging (MRI), computed tomography (CT) scan or sonography), preoperative 24-h urine catecholamine levels (which were recorded as a level of 24-h urine metanephrine, normetanephrine, epinephrine, norepinephrine, and vanillymandelic acid (VMA)), preoperative medications, PhB cumulative dose taken in mg and dose taken per day in mg/day, days before surgery that PhB was administered and normal saline serum administered or not, BP on admission and right before surgery detected in the operating room.
Preoperative medications included prazosin, propranolol, metoprolol, amlodipine, and ACEIs or ARBs. We recorded prazosin, propranolol, and metoprolol in mg. About ACEIs, ARBs and amlodipine, we just mentioned whether they were administered or not.
The normal ranges for 24-h urine metanephrine, normetanephrine, epinephrine, norepinephrine, and VMA were < 350, < 600, < 10, 14 - 80, and < 13.6 µg/24 h, respectively. These are normal ranges of detecting kits of laboratories in Isfahan.
We included the maximum tumor size that has been recorded by imaging reports.
Intraoperative and surgery-related data included: type of surgery (open or laparoscopic), HTC during surgery, a hypotensive drug administered (we just recorded if it was prescribed or not), and during surgery BP.
Postoperative data included: postoperative BP and pathology of the tumor.
In this study, HTC during surgery was defined as an SBP > 180 mm Hg and/or the need for hypotensive agent infusion. Postoperative hypotension was defined as an SBP < 90 mm Hg. Hypotension during surgery was defined as BP < 100 mm Hg [28, 29]. It is considerable that all patients took PhB on the morning of surgery.
We prepared the main data of all patients in Table 1.
 Click to view | Table 1. Demographic and Therapeutic Details of the Patients |
Statistical analysis
SPSS (Statistical Package for the Social Sciences V. 16, IBM Corporation) was used for statistical analysis. The quantitative and categorical data were expressed as mean ± standard deviation and frequency and percentage, respectively. Statistical analysis was performed by Chi-squared or Fisher’s exact tests to compare categorical variables while the Mann-Whitney U test was used to compare continuous variables between groups. Variables with P < 0.1 in the univariate analysis were entered into multivariable logistic regression analysis (binary logistic regression), and odds ratio (OR), and 95% confidence interval (CI) for OR were reported. Repeated measures analysis of variance (ANOVA) was used for evaluating and comparing the change in BP (SBP and diastolic blood pressure (DBP)) in each group and between two groups during admission, before, during, and after surgery. We also used the receiver operating characteristic (ROC) curve to examine the best cutoff value of tumor size and reported the sensitivity, specificity, and positive and negative predictive values (PPV, NPV) along with the area under the curve (AUC) with a 95% CI. P ≤ 0.05 was chosen as statistically significant.
| Results | ▴Top |
The mean age of participants was 45.2 ± 13.5 years and 30 (57.7%) of the patients were women. The mean PhB cumulative dose was 570.19 ± 465.25 mg and the mean daily dose was 30.34 ± 27.85 mg/day. The mean time of taking PhB was 19.7 ± 16.5 days.
With doses of less than 20 mg/day, 24-h normetanephrine mean level was 410.10 ± 341.39 µg/24 h, with 20 - 35 mg/day, it was 799.20 ± 452.71 µg/24 h and with doses more than 35 mg/day, it was 1,892.43 ± 1,116.05 µg/24 h (P = 0.007). However, mean levels of 24-h urine metanephrine, epinephrine, norepinephrine, and VMA were not different (P > 0.05). With the SBPs less than 130 mm Hg, the mean dose was 26.71 ± 12.35 mg/day, and in BPs from 130 to 160 mm Hg, it was 34.61 ± 16.50 mg/day and for SBP more than 160 mm Hg, mean dose was 42.16 ± 13.16 mg/day (P = 0.039). The dose was not different in groups of tumor sizes > 33.5 mm and tumor sizes < 33.5 mm (P > 0.05).
No patient experienced hypotension during the operation or postoperatively. There were no perioperative deaths either.
Prazosin was administered in 14 patients (26.9%). Seven of them (50%) took it because PhB was not available at first. When it became available, taking prazosin was stopped. It was prescribed at least 10 days before surgery. Forty-six patients (88.5%) took propranolol or metoprolol in preparation for the surgery. Four (8.7%) patients took it 1 day before the surgery and for others, it was administered at least 2 days before surgery. The mean time to prescribe β-blocker was 9.2 ± 11.3 days. Twenty-two patients (42.3%) and 15 patients (28.8%) took amlodipine and ACEIs/ARBs respectively before the surgery. Normal saline serum was prescribed for patients to be prepared for the operation but seven patients (13.5%) did not take any normal saline. The mean time to prescribe normal saline was 7.4 ± 6.3 days.
Twelve patients (23.1%) experienced HTC during surgery. There was no significant difference in terms of HTC during surgery and history of ischemic heart disease, hypertension, diabetes mellitus, hypothyroidism, neurofibromatosis, MEN2, and previous pheochromocytoma (P > 0.05). ROC analysis results showed tumor sizes more than 33.5 mm differentiate significantly in patients with HTC during surgery from non-HTC (P = 0.023, sensitivity = 91.7%, specificity = 42.5%, NPV = 94.1%, PPV = 31.4%, AUC: 0.667, CI: 0.499 - 0.835) (Fig. 1). There was no statistically significant difference in terms of HTC and days to prepare patients (P = 0.447) (did have HTC: 16.5 ± 9.6 days vs. did not have HTC: 20.7 ± 18.0 days). We found out that patients who had HTC during surgery had higher BPs on all points (Figs. 2, 3). Pheochromocytoma panel (including 24-h urine metanephrine, normetanephrine, epinephrine, norepinephrine, and VMA) was not different between HTC and non-HTC groups (P > 0.05). We did not see any difference in the side of the tumor (right, left, or bilateral) and type of surgery between these two groups either (P > 0.05) (Table 2).
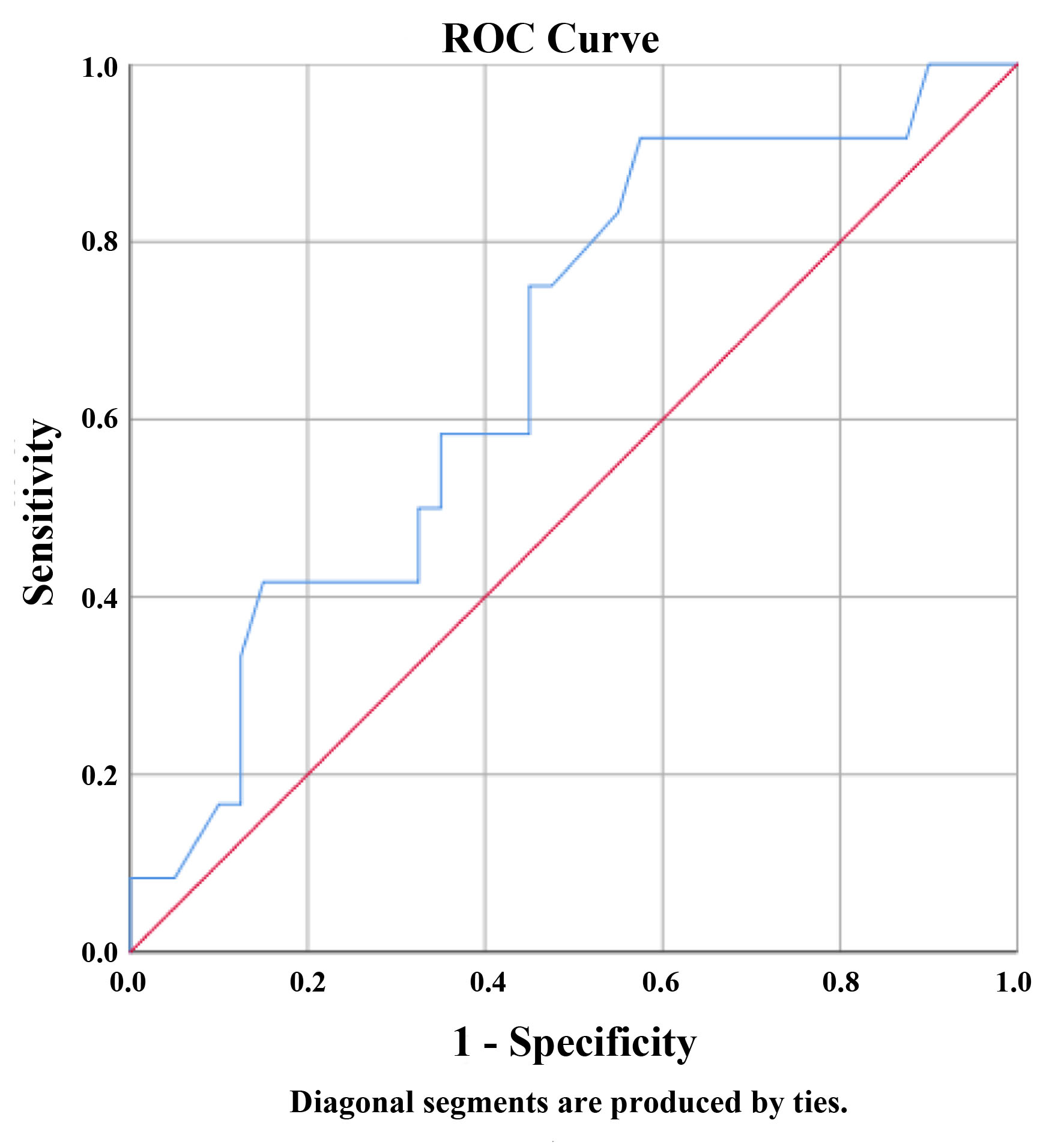 Click for large image | Figure 1. Receiver operating characteristic (ROC) curve of hypertensive crisis during surgery by tumor size reported by imaging. The size of the tumor in mm has at least one tie between the positive actual state group and the negative actual state group. Area: 0.667, P = 0.082, 95% confidence interval: 0.499 - 0.835. |
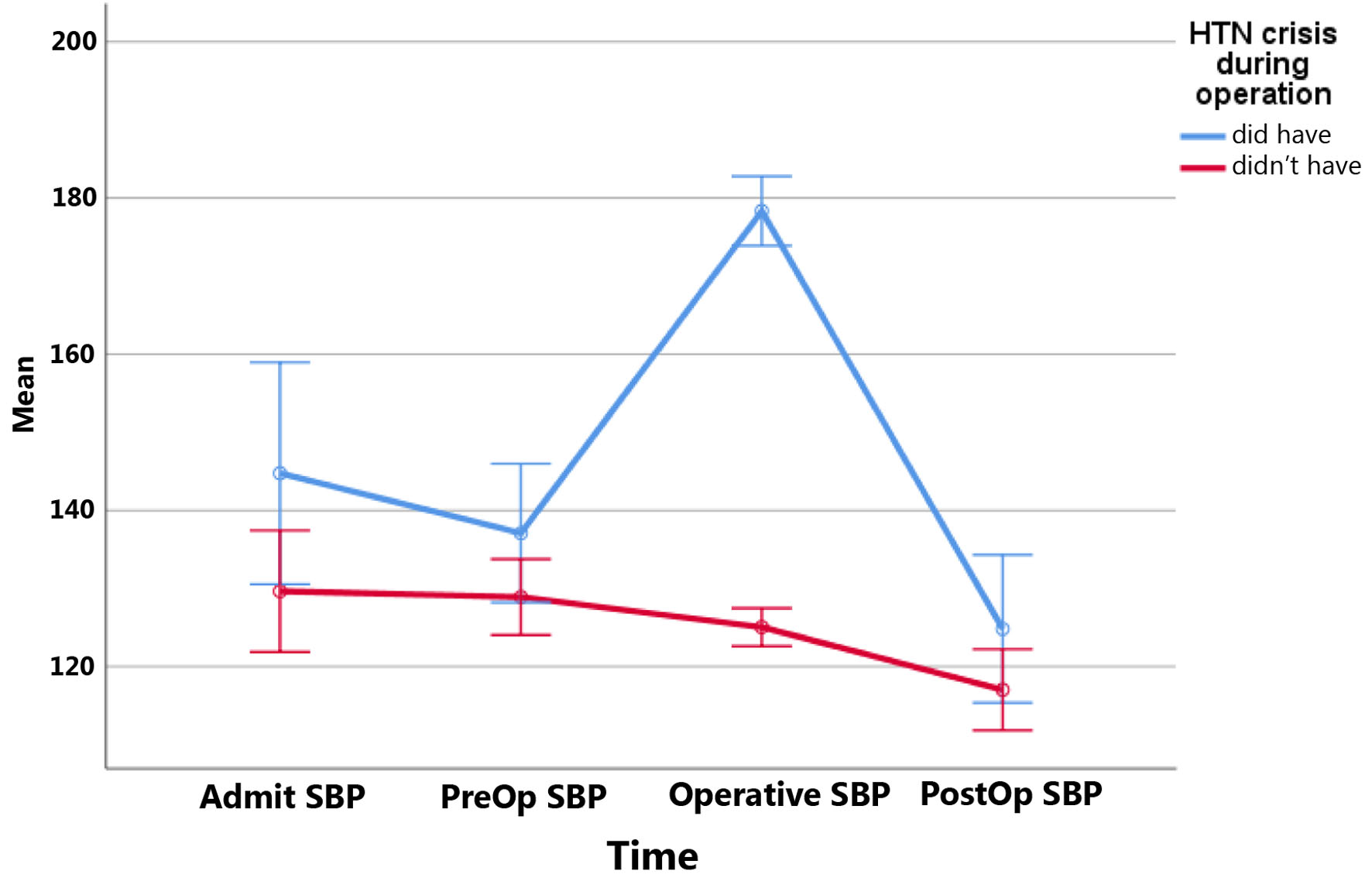 Click for large image | Figure 2. Systolic blood pressures (SBPs) at different times in patients with and without hypertensive crisis during surgery. Mean SBPs at different times: SBP on admission; PreOp: on the day of surgery; Operative: in the operative room during surgery; PostOp: postoperatively. HTN: hypertension. |
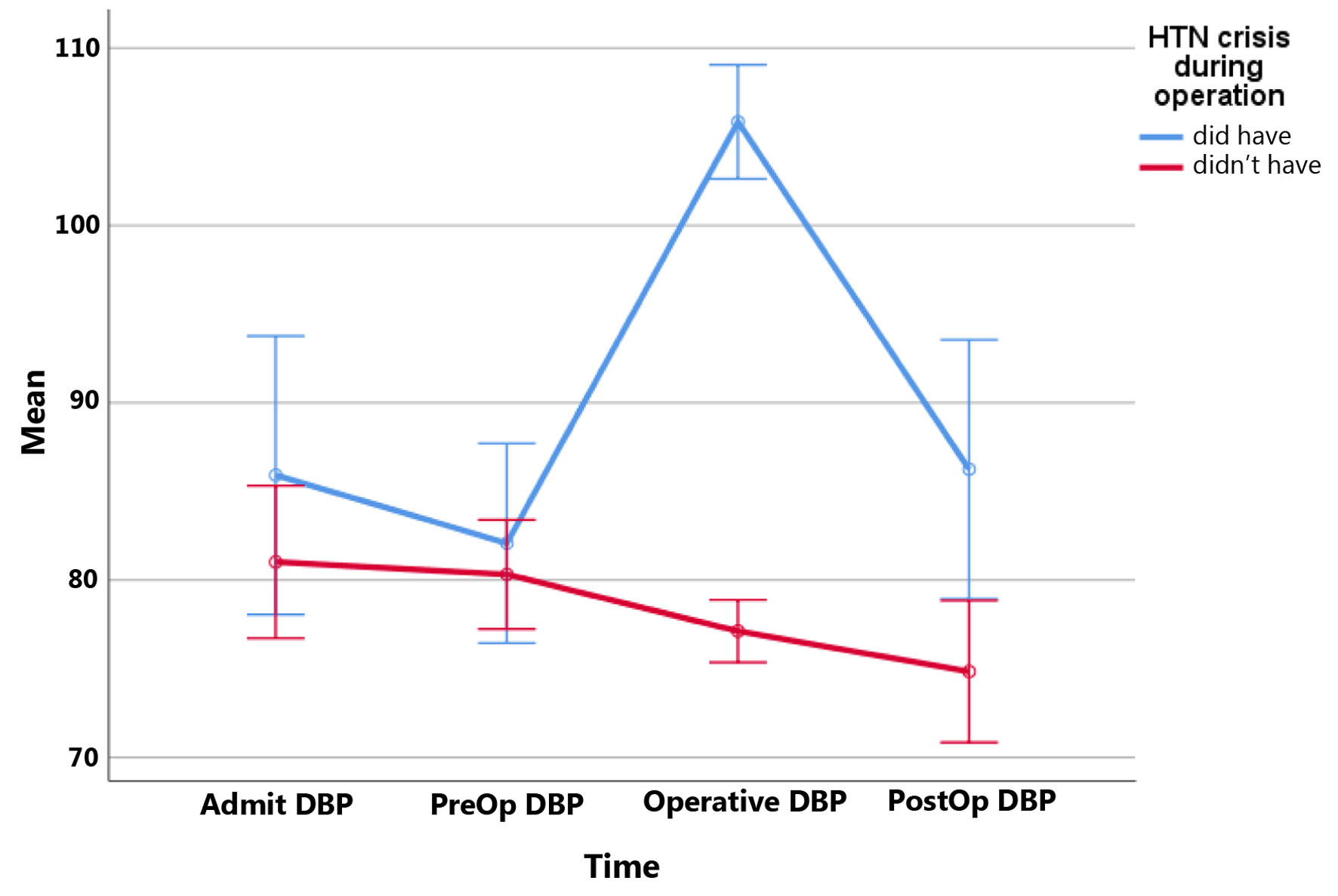 Click for large image | Figure 3. Diastolic blood pressures (DBPs) at different times in patients with and without hypertensive crisis during surgery. Mean DBPs at different times: DBP on admission; PreOp: on the day of surgery; Operative: in the operative room during surgery; PostOp: postoperatively. HTN: hypertension. |
 Click to view | Table 2. Risk Factors of Hypertensive Crisis During Surgery |
Those variables associated with HTC during surgery with a P < 0.1 (Table 2) in univariate analysis were entered in multivariable binary logistic regression. Results showed that patients with tumor sizes of more than 33.5 mm are 13.1 times more likely to experience HTC during surgery (OR = 13.1, P = 0.031). Patients who took amlodipine as a help to decrease their BPs were 5.1 times (OR = 5.1, P = 0.05) more likely to experience HTC during surgery (Table 3).
 Click to view | Table 3. Logistic Regression Analysis for Predicting HTC During Surgery |
| Discussion | ▴Top |
Adrenalectomy is the recommended method to treat pheochromocytoma [30-32]. The important note is that patients are at risk of hemodynamic instability before, during, and after surgery [31]. Therefore, appropriate preparation for the surgery is necessary to prevent complications [31].
In the Azahra Hospital of Isfahan, endocrinologists tend to choose PhB to prepare the patients. It provides more preventive effects by irreversible binding to receptors, more immediate effects, and a significant hypotensive effect [33]. The use of the drug could greatly reduce intraoperative hemodynamic instability and patient death during surgery [15].
The initial PhB dose is once to twice daily, usually 10 mg, with an increase of 10 - 20 mg in divided doses every 2 - 3 days. Most commonly, the patient is ready for the surgery within the first 7 - 14 days after initiation of PhB. The final dose is most typically between 20 and 100 mg daily [34]. This was the chosen approach to prepare the patients in the Azahra Hospital, too.
In our center, the mean time of preoperative management was 19.7 ± 16.5 days with a median of 14.0 days. However, researchers have reported median duration of 27.4 days, 16 days, 35 days, 38.8 days, and even 14 weeks [10, 29, 35-37]. Our study, like most previous studies, defined the duration of preoperative management from the time of beginning to surgery [28, 34, 35]. We did not find any difference between this duration and the group of patients with and without hemodynamic alteration during surgery (P = 0.601), like the studies Russel et al, Kiernan et al and Liu et al did [36, 38, 39]. Russel et al reviewed 14 patients’ data who underwent adrenalectomy in a retrospective study from 1980 to 1993 and concluded that longer treatment with PhB cannot cause better perioperative BP stability [38]. In Liu et al’s study, a total of 253 patients were grouped according to their tumor diameter: diameters of ≥ 8 cm, 6 to 8 cm, and < 6 cm. They showed that there was no difference in the time of preparation for these three groups of patients [39]. It suggests that spending long days preparing patients for surgery is not necessary and helpful [36, 38, 39]. We should mention that all these studies used imaging reports to do analyses on tumor size.
In a study, the mean PhB dose was 29.4 (20 - 40) mg/day [37]. In this study, Tian et al studied 102 patients’ data who were diagnosed with pheochromocytoma and received surgical treatment from 2001 to 2018 in China. They took PhB from admission in the hospital and they mentioned this mean dose. It was close to our study with a mean dose of 30.34 ± 14.22 mg/day (P = 0.635). There was no difference in PhB dose between groups of tumor size or groups of patients with and without hemodynamic instability during surgery in their study. In some other studies, mean doses were 55.0 ± 22.2 and 40 ± 23 mg/day [29, 40]. There was no difference in PhB dose between tumor sizes > 33.5 mm and tumor sizes ≤ 33.5 mm groups in our study (P > 0.05). In Liu et al’s study, larger tumors needed more PhB dose [39]. Because pheochromocytoma is a rare disease and most studies are retrospective, we do not know if determining the dose of the drug to prepare patients depends solely on measuring BP or if some endocrinologists prefer to prescribe higher doses of PhB for larger tumors. They increase the dose of the drug until it is tolerable. Therefore, perhaps comparing the dose of the drug between different studies only indicates the prescriptions of each physician according to his/her experiences.
We also found that patients with admitted SBPs of more than 130 mm Hg needed more doses of PhB. A study, that Miahi and colleagues did in 2008, showed that there was no correlation between this variable and the dose of PhB [29]. Their study was a retrospective review of 60 patients with pheochromocytoma who underwent adrenalectomy after adrenergic blockade using a standardized protocol between 1998 and 2007. Therefore, we suggest a new item to pay attention to managing pheochromocytoma. However, more studies are needed to confirm this finding.
We noticed that patients with higher levels of 24-h urine normetanephrine needed more PhB. In the study that Mihai et al did [29], there was no significant correlation between the dose of PhB and levels of 24-h urine normetanephrine. Maybe this metabolite could be a predictor of more PhB needed to prepare patients for the surgery.
We also found that with more 24-h urine metanephrine levels, a bigger tumor can be expected, like the study Carr et al did [41]. They studied 70 patients’ data retrospectively and concluded that urine normetanephrine, metanephrine, and norepinephrine levels were correlated with bigger sizes of the tumor.
Our study showed that despite adequate treatment with PhB and other prescriptions before surgery, HTC was not uncommon (12 patients (23.1%)). Therefore, it is of great importance to see why patients experienced HTC during adrenalectomy despite this management.
In our study, larger tumors resulted in a significant increase in the HTC during surgery (a larger maximal diameter of > 33.5 mm reported by imaging, P = 0.023). It was consistent with other studies [22, 36, 42-45]. However, HTC during the operation was not correlated with catecholamine levels alone. This may suggest that the intraoperative manipulation of larger tumors causes a release of larger amounts of catecholamines than are recorded at baseline, which is consistent with Kiernan et al’s conclusion of their study [36]. In another study, Bruynzeel et al analyzed data from 73 patients who underwent operation from 1995 to 2007 in a retrospective pattern. They concluded that larger tumor size is a risk factor for hemodynamic instability during surgery (> 4 cm, r = 0.27, P < 0.05) [22]. In a retrospective study that Scholten and colleagues did, 61 pheochromocytoma resections between 2000 and 2010 were done. They concluded that tumor size is an independent risk factor for HTC during surgery (> 3 cm, r = 0.39, P = 0.002) [44].
There was no difference in the type of surgery between groups of patients with and without HTC during surgery. It is consistent with Davies et al’s study [46]. They compared 24 patients’ data, in which 12 patients underwent open and 12 patients underwent laparoscopic surgery. They concluded that there was no statistically significant difference between the two groups. However, Kiernan and colleagues studied 91 patients’ records and concluded that open surgery was associated with HTC during surgery [36].
As mentioned earlier, we had 12 patients with HTC during surgery. Four of them (33.3%) received PhB less than 20 mg/day. All of them had our criteria of preparation for the surgery but six of them (50%) had them right before surgery with SBP > 130 mm Hg. For four patients, we could not find a reason. However, three of them (75%) had tumor sizes greater than 50 mm. The other one’s tumor size was 34 mm. It is considerable that all 52 patients were prepared and then were sent to the operating room and these BPs before surgery were detected by anesthesiologists.
As mentioned above, all BPs before surgery in patients with HTC were greater than in patients without this crisis. A study that Buitenwerf et al did showed that a preoperative SBP < 130 mm Hg is associated with less hemodynamic instability during surgery [47]. They did a randomized controlled trial from 2012 to 2017 in the Netherlands on a total of 144 patients and concluded this result about preoperative SBP but they did not mention SBP on admission. It shows that maybe lowering these BPs could help prevent HTC during surgery. So increased dose of PhB to decrease these BPs to SBPs < 130 mm Hg can be a significant predictor of improved intraoperative hemodynamic stability. This conclusion is parallel with the study which Livingstone et al did [8]. They reviewed 100 pheochromocytoma resections from 1992 to 2013 and concluded that increased preoperative doses of PhB over the last 10 years of practice have likely contributed to the improvements in perioperative outcomes.
This issue that taking anti-hypertensive drugs aside from α- and β-blockers, including ACEIs and ARBs or amlodipine, could predict an HTC during surgery was analyzed. We found that patients taking amlodipine to control BP were at more risk for HTC during surgery. Amlodipine, a long-acting calcium channel blocker, is mainly used to supplement α-blockers in patients with poor BP control, to obviate the need of increasing the dosage of α-blockers, to replace α-blockers when causing severe side effects, and to prevent α-blocker-induced sustained hypotension in patients with only intermittent hypertension. Calcium channel blockers do not cause hypotension or orthostatic hypotension when a patient is normotensive [20]. We did not find a study to focus on this variable. We suggest more clinical trial studies to evaluate this drug and its effect on HTC during surgery but our recommendation is to reduce the use of amlodipine and instead, more α-blockers be used as possible as they can be used while preparing the patients for adrenalectomy and more hydration and high sodium diet be administered to prevent probable hypotension of patients.
There were several limitations in our study. First, this was a retrospective study spanning 9 years and any retrospective study is limited by the quality and inaccuracy of data reporting. Second, the analysis of HTC during surgery was limited by the small number of patients who experienced this crisis during surgery (12 patients, 23.1%). Third, it is the fact that it was a single-center study. Fourth, because of incomplete data recording, we could not record patients’ body mass index (BMI) and therefore, it may have affected some of our data analyses. Finally, our center was a COVID-19 referral center when the epidemic started, so most surgeries could not be done, which caused a limitation in the sample of our study.
Despite these limitations, we could find some analyses that few studies had mentioned, like taking amlodipine and its predictor value of HTC during surgery. One of the strengths of the study was the relatively good number of patients. Also, it is worth mentioning that this study is the only study that is done in Iran, and therefore its data can be helpful for the next studies.
Conclusion
This study shows that for patients with tumor sizes of > 33.5 mm, it is more probable to cause HTC during surgery. So probably the nature of the tumor has something to do with catecholamine secreting process. Patients taking amlodipine to control BP were at more risk for HTC during surgery, too. More studies are needed to evaluate this issue but our recommendation is to reduce the use of amlodipine and instead, more PhB be used as much possible as it can be used while preparing the patients.
Also, all SBPs before surgery in patients with HTC were greater than in patients without HTC. It shows that maybe lowering these SBPs could help prevent HTC during surgery. Therefore, increasing doses of PhB to decrease the SBPs to < 130 mm Hg can be a significant predictor of improved intraoperative hemodynamic stability.
Acknowledgments
We gratefully thank Isfahan University of Medical Sciences and Tehran University of Medical Sciences for the support.
Financial Disclosure
This work was supported solely by institutional and departmental sources.
Conflict of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Informed Consent
Not applicable.
Author Contributions
Mozhgan Karimifar: writing and edition, suggesting the idea and responsible for conceptualization. Sina Abbaspour: writing and data collection and statistical analysis. Awat Feizi: statistical analysis and edition. Mitra Heidarpour: data collection and edition.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Randle RW, Balentine CJ, Pitt SC, Schneider DF, Sippel RS. Selective versus non-selective alpha-blockade prior to laparoscopic adrenalectomy for pheochromocytoma. Ann Surg Oncol. 2017;24(1):244-250.
doi pubmed - Tischler AS, de Krijger RR. Phaeochromocytoma. In: Lloyd RV, Osamura RY, Kloppel G, editors. WHO Classification of Tumors of Endocrine Organs. 4th ed. IARC Press; Lyons, France: 2017. p. 183-189.
- van der Horst-Schrivers AN, Kerstens MN, Wolffenbuttel BH. Preoperative pharmacological management of phaeochromocytoma. Neth J Med. 2006;64(8):290-295.
- Kinney MA, Narr BJ, Warner MA. Perioperative management of pheochromocytoma. J Cardiothorac Vasc Anesth. 2002;16(3):359-369.
doi pubmed - Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal-time for a reappraisal? Eur J Endocrinol. 2011;165(3):365-373.
doi pubmed - Soltani A, Pourian M, Davani BM. Does this patient have Pheochromocytoma? a systematic review of clinical signs and symptoms. J Diabetes Metab Disord. 2015;15:6.
doi pubmed - Baguet JP, Hammer L, Mazzuco TL, Chabre O, Mallion JM, Sturm N, Chaffanjon P. Circumstances of discovery of phaeochromocytoma: a retrospective study of 41 consecutive patients. Eur J Endocrinol. 2004;150(5):681-686.
doi pubmed - Livingstone M, Duttchen K, Thompson J, Sunderani Z, Hawboldt G, Sarah Rose M, Pasieka J. Hemodynamic stability during pheochromocytoma resection: lessons learned over the last two decades. Ann Surg Oncol. 2015;22(13):4175-4180.
doi pubmed - Bajwa SS, Bajwa SK. Implications and considerations during pheochromocytoma resection: A challenge to the anesthesiologist. Indian J Endocrinol Metab. 2011;15 Suppl 4:S337-344.
doi pubmed - Malec K, Miskiewicz P, Witkowska A, Krajewska E, Toutounchi S, Galazka Z, Piotrowski M, et al. Comparison of phenoxybenzamine and doxazosin in perioperative management of patients with pheochromocytoma. Kardiol Pol. 2017;75(11):1192-1198.
doi pubmed - Ludwig AD, Feig DI, Brandt ML, Hicks MJ, Fitch ME, Cass DL. Recent advances in the diagnosis and treatment of pheochromocytoma in children. Am J Surg. 2007;194(6):792-796; discussion 796-797.
doi pubmed - Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346(19):1459-1466.
doi pubmed - Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, Buchta M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292(8):943-951.
doi pubmed - Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3(2):92-102.
doi pubmed - Zhu Y, He HC, Su TW, Wu YX, Wang WQ, Zhao JP, Shen Z, et al. Selective alpha1-adrenoceptor antagonist (controlled release tablets) in preoperative management of pheochromocytoma. Endocrine. 2010;38(2):254-259.
doi pubmed - Costa Almeida CE, Silva M, Carvalho L, Costa Almeida CM. Adrenal giant cystic pheochromocytoma treated by posterior retroperitoneoscopic adrenalectomy. Int J Surg Case Rep. 2017;30:201-204.
doi pubmed - Babic B, Patel D, Aufforth R, Assadipour Y, Sadowski SM, Quezado M, Nilubol N, et al. Pediatric patients with pheochromocytoma and paraganglioma should have routine preoperative genetic testing for common susceptibility genes in addition to imaging to detect extra-adrenal and metastatic tumors. Surgery. 2017;161(1):220-227.
doi pubmed - Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942.
doi pubmed - Inchiosa MA, Jr. Anti-tumor activity of phenoxybenzamine and its inhibition of histone deacetylases. PLoS One. 2018;13(6):e0198514.
doi pubmed - Garg MK, Kharb S, Brar KS, Gundgurthi A, Mittal R. Medical management of pheochromocytoma: Role of the endocrinologist. Indian J Endocrinol Metab. 2011;15 Suppl 4:S329-336.
doi pubmed - Young WF, Kebebew E. Treatment of pheochromocytoma in adults. In: Nieman LK, Carty SE (Eds.). UpToDate. 2018.
- Bruynzeel H, Feelders RA, Groenland TH, van den Meiracker AH, van Eijck CH, Lange JF, de Herder WW, et al. Risk factors for hemodynamic instability during surgery for pheochromocytoma. J Clin Endocrinol Metab. 2010;95(2):678-685.
doi pubmed - Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison's principles of internal medicine, 19th ed. [monograph on the Internet]. New York: The McGraw-Hill Companies, Inc.; 2015.
- Conzo G, Musella M, Corcione F, Depalma M, Stanzione F, Della-Pietra C, Palazzo A, et al. Role of preoperative adrenergic blockade with doxazosin on hemodynamic control during the surgical treatment of pheochromocytoma: a retrospective study of 48 cases. Am Surg. 2013;79(11):1196-1202.
doi pubmed - Kim HH, Kim GH, Sung GT. Laparoscopic adrenalectomy for pheochromocytoma: comparison with conventional open adrenalectomy. J Endourol. 2004;18(3):251-255.
doi pubmed - Kim JH, Lee HC, Kim SJ, Yoon SB, Kong SH, Yu HW, Chai YJ, et al. Perioperative hemodynamic instability in pheochromocytoma and sympathetic paraganglioma patients. Sci Rep. 2021;11(1):18574.
doi pubmed - Dluhy RG, Lawrence JE, Williams GH. Larsen: Williams Textbook of Endocrinology. 14th ed. Saunders; 2019. Pheochromocytoma.
- van der Zee PA, de Boer A. Pheochromocytoma: a review on preoperative treatment with phenoxybenzamine or doxazosin. Neth J Med. 2014;72(4):190-201.
- Mihai R, Sadler GP, Bridge H. Adrenergic blockade with phenoxybenzamine and propranolol in a cohort of 60 patients undergoing surgery for phaeochromocytoma. Eur J Anaesthesiol. 2008;25(6):508-510.
doi pubmed - Mannelli M. Management and treatment of pheochromocytomas and paragangliomas. Ann N Y Acad Sci. 2006;1073:405-416.
doi pubmed - Li J, Yang CH. Improvement of preoperative management in patients with adrenal pheochromocytoma. Int J Clin Exp Med. 2014;7(12):5541-5546.
- Conzo G, Musella M, Corcione F, De Palma M, Ferraro F, Palazzo A, Napolitano S, et al. Laparoscopic adrenalectomy, a safe procedure for pheochromocytoma. A retrospective review of clinical series. Int J Surg. 2013;11(2):152-156.
doi pubmed - Weingarten TN, Cata JP, O'Hara JF, Prybilla DJ, Pike TL, Thompson GB, Grant CS, et al. Comparison of two preoperative medical management strategies for laparoscopic resection of pheochromocytoma. Urology. 2010;76(2):508.e506-511.
doi pubmed - Yoham AL, Casadesus D. Phenoxybenzamine. [Updated 2021 Jul 23]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Goldstein RE, O'Neill JA, Jr., Holcomb GW, 3rd, Morgan WM, 3rd, Neblett WW, 3rd, Oates JA, Brown N, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-764; discussion 764-756.
doi pubmed - Kiernan CM, Du L, Chen X, Broome JT, Shi C, Peters MF, Solorzano CC. Predictors of hemodynamic instability during surgery for pheochromocytoma. Ann Surg Oncol. 2014;21(12):3865-3871.
doi pubmed - Tian J, Bao Z, Yuan Y, Fang D, Zhan Y, Wang T, Zhang Z, et al. The duration of preoperative administration of single alpha-receptor blocker phenoxybenzamine before adrenalectomy for pheochromocytoma: 18 years of clinical experience from nationwide high-volume center. Biomed Res Int. 2019;2019:2613137.
doi pubmed - Russell WJ, Metcalfe IR, Tonkin AL, Frewin DB. The preoperative management of phaeochromocytoma. Anaesth Intensive Care. 1998;26(2):196-200.
doi pubmed - Liu H, Li B, Yu X, Huang Y. Perioperative management during laparoscopic resection of large pheochromocytomas: A single-institution retrospective study. J Surg Oncol. 2018;118(4):709-715.
doi pubmed - Falhammar H, Kjellman M, Calissendorff J. Treatment and outcomes in pheochromocytomas and paragangliomas: a study of 110 cases from a single center. Endocrine. 2018;62(3):566-575.
doi pubmed - Carr JC, Spanheimer PM, Rajput M, Dahdaleh FS, Lal G, Weigel RJ, Sugg SL, et al. Discriminating pheochromocytomas from other adrenal lesions: the dilemma of elevated catecholamines. Ann Surg Oncol. 2013;20(12):3855-3861.
doi pubmed - Kinney MA, Warner ME, vanHeerden JA, Horlocker TT, Young WF, Jr., Schroeder DR, Maxson PM, et al. Perianesthetic risks and outcomes of pheochromocytoma and paraganglioma resection. Anesth Analg. 2000;91(5):1118-1123.
doi pubmed - Guerrero MA, Schreinemakers JM, Vriens MR, Suh I, Hwang J, Shen WT, Gosnell J, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009;209(6):727-732.
doi pubmed - Scholten A, Vriens MR, Cromheecke GJ, Borel Rinkes IH, Valk GD. Hemodynamic instability during resection of pheochromocytoma in MEN versus non-MEN patients. Eur J Endocrinol. 2011;165(1):91-96.
doi pubmed - Araujo-Castro M, Garcia Centeno R, Lopez-Garcia MC, Lamas C, Alvarez-Escola C, Calatayud Gutierrez M, Blanco-Carrera C, et al. Risk factors for intraoperative complications in pheochromocytomas. Endocr Relat Cancer. 2021;28(11):695-703.
doi pubmed - Davies MJ, McGlade DP, Banting SW. A comparison of open and laparoscopic approaches to adrenalectomy in patients with phaeochromocytoma. Anaesth Intensive Care. 2004;32(2):224-229.
doi pubmed - Buitenwerf E, Osinga TE, Timmers H, Lenders JWM, Feelders RA, Eekhoff EMW, Haak HR, et al. Efficacy of alpha-blockers on hemodynamic control during pheochromocytoma resection: a randomized controlled trial. J Clin Endocrinol Metab. 2020;105(7):2381-2391.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
