| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 1, Number 2, June 2011, pages 73-78
Serum Levels of B-Cell Activating Factor of TNF Family (BAFF) as a Useful Indicator for the Activity of Graves’ Disease
Sumito Sunagawaa, Tsuyoshi Koukib, Shin-ichiro Tairaa, Rei Uedaa, Kouichi Yabikua, Tomomi Ikemaa, Ayako Nakachia, Chisayo Kozukaa, Moritake Higac, Ken Yamakawaa, Michio Shimabukurod, Hiroaki Masuzakia, e
aDivision of Endocrinology, Diabetes and Metabolism, Hematology, Rheumatology (Second Department of Internal Medicine), Graduate School of Medicine, University of the Ryukyus, Okinawa, Japan
bNakamura Clinic, Okinawa, Japan
cDiabetes and Life-style Related Disease Center, Tomishiro Chuo Hospital, Okinawa, Japan
dDepartment of Cardio-Diabetes Medicine, University of Tokushima Graduate School of Health Biosciences, Tokushima, Japan
eCorresponding author: Hiroaki Masuzaki, 207 Uehara Nishihara Okinawa, Japan
Manuscript accepted for publication May 25, 2011
Short title: Serum BAFF Level and Activity of Graves’ Disease
doi: https://doi.org/10.4021/jem16w
| Abstract | ▴Top |
Background: Graves’ disease (GD) is a thyroid-specific autoimmune disorder. Both B-cell activating factor of TNF family (BAFF) and a proliferation-inducing ligand (APRIL) are cytokines which share a role for regulating survival and proliferation of B lymphocytes. Elevated level of these cytokines in circulation was observed in several types of autoimmune diseases. However, there were no reports focusing on the potential relationship between BAFF and the activity of GD. To explore the pathophysiologic role of BAFF and APRIL in GD, we investigated serum levels of BAFF and APRIL in patients with GD.
Methods: We enrolled twenty three patients newly diagnosed as GD and twenty healthy donors as controls. We measured serum levels of BAFF and APRIL in patients and controls by ELISA.
Results: Serum level of BAFF was significantly elevated in patients with GD compared to controls (1329 ± 435 pg/mL vs. 983 ± 308 pg/mL, P < 0.01). Notably, serum BAFF level was significantly correlated with free-T3 to free-T4 ratio (FT3/FT4), a useful indicator for the disease activity of GD.
Conclusions: Findings in the present study offer a novel opportunity to evaluate GD.
Keywords: Cytokine; BAFF; APRIL; Graves’ disease; Autoimmunity; Free T3 to free T4 ratio
| Introduction | ▴Top |
Graves’ disease (GD) is a thyroid-specific autoimmune disorder. GD predominantly involves lymphocytic, tissue-specific invasion of the thyroid gland, leading to T cell-dependent antibody production from B cell against thyroid antigens [1].
B-cell activating factor of tumor necrosis factor (TNF) family (BAFF), also known as B lymphocyte stimulator (BLyS), is considerably expressed in monocytes, macrophages and dendritic cells (DCs), and in T cells at a lower level. BAFF is a potent cytokine by which activated macrophages and DCs regulate B cell function in humans [2, 3]. BAFF specifically binds to three members of the TNF receptor superfamily including BAFF receptor expressed in B cells. BAFF is also a potent modulator of B cell survival and differentiation [4].
Exaggerated activation of B cell and T cell by BAFF results in developing and/or worsening autoimmune diseases. Transgenic (Tg) mice overexpressing BAFF show an expansion of peripheral mature B cell compartment, hyperglobulinemia, antibodies against single-stranded and double-stranded DNAs, and circulating immune complexes. The Tg mice develop manifestations similar to human systemic lupus erythematosus (SLE) and primary Sjogren syndrome (pSS) [5]. Although BAFF is a type II transmembrane protein, it is secreted as a soluble form after the cleavage from the cell membrane [4]. Evidence is accumulated that serum BAFF level was elevated in patients with rheumatoid arthritis, where the value of circulating BAFF was correlated with IgG level [6]. Serum BAFF level was also elevated in patients with SLE, partially associating with higher level of anti-dsDNA antibody of the IgG, IgM, and IgA classes [7]. Salivary gland epithelial cells in patients with pSS also express BAFF [8].
On the other hand, a proliferation-inducing ligand (APRIL) is a close homolog to BAFF expressing in monocytes, macrophages, DCs and T cells [9, 10]. APRIL exists as a secreted soluble form [4]. It is likely that exaggerated activation of B cell and T cell by APRIL also results in developing and worsening autoimmune disorder. A recent report demonstrated that serum APRIL level was elevated significantly in patients with SLE [11]. Plasma APRIL level in patients with idiopathic thrombocytopenia (ITP) was significantly elevated and returned to the normal level in the course of treatment [12].
Taken together, serum BAFF and APRIL level would be useful for estimating the activity of a line of autoimmune diseases. Furthermore, BAFF and APRIL attract attention on cytokine targeting therapy by monoclonal autoantibody. As a matter of fact, recent studies demonstrated that inhibition of BAFF or simultaneous blockade of BAFF and APRIL in patients with rheumatoid arthritis and systemic lupus erythematosus reduced disease activity, accompanied by a decrease in immunoglobulin levels [13].
Fabris et al. [14] showed that serum BAFF level was elevated in patients with GD and Hashimoto’s thyroiditis. However, there have been no reports exploring the potential relationship between BAFF/APRIL and the disease activity in autoimmune thyroid diseases. In this context, the present study was designed to investigate the possible clinical link between the disease activity of GD and circulating level of BAFF or APRIL.
| Materials and Methods | ▴Top |
Participants
In the present study, we enrolled twenty three patients who were newly diagnosed as GD and twenty healthy donors (HD) (Table 1). GD patients included fourteen females and nine males (mean age 40.7 ± 18.4 years). GD patients were age- and sex-matched with HD. There were no diagnoses of other autoimmune disease or infection when they were diagnosed GD. The diagnosis of GD was made on the basis of clinical features including diffuse goiter, exophthalmos, tachycardia, palpitation, tremor and sweating as well as laboratory data including undetectable level of serum thyroid stimulating hormone (TSH), elevated levels of serum free-thyroxine (FT4) and thyrotropin receptor antibody (TRAb).
 Click to view | Table 1. Clinical Characteristics of the Subjects Studied |
Measurement of hormone and cytokine levels
Serum levels of TSH, FT3, and FT4 were measured by ECLIA (SRL, Tokyo, Japan). Normal range of TSH was 0.500 - 5.00 µIU/mL, and lower detection limit was 0.01µIU/mL. Normal ranges of FT3 and FT4 were 2.30 - 4.30 pg/mL and 0.90 - 1.70 ng/dL respectively, and higher detection limits were 25.0 pg/mL and 7.77 ng/dL respectively. TRAb was measured as TSH-binding inhibitory immunoglobulin (TBII) and thyroid-stimulating antibodies (TSAb). TBII was measured by ELISA kit (RSR Ltd, Cardiff, UK, SRL, Tokyo, Japan). Serum levels of BAFF and APRIL were measured by ELISA kits (BAFF: Quantikine Human BAFF/BLyS, R&D Systems, Minneapolis, MN, USA – lower limit of detection: 3.38 pg/mL; APRIL: Human APRIL ELISA, Bender Med System, Wien, Austria – lower limit of detection: 780 pg/mL). Moreover, it has been demonstrated that helper T1 (Th1) pattern of immune response, a characteristic of cellular immunity, is dominant in Hashimoto’s thyroiditis, whereas the predominance of helper T2 (Th2) cytokines in GD indicates a humoral pattern of immune reaction [15, 16]. In this context, to gain further insight into BAFF and APRIL in the pathophysiology of GD, we also measured circulating levels of cytokines associated with Th1 or Th2 cells including IFN-γ (Th1 cytokines) and IL-4 or IL-13 (Th2 cytokines). Serum levels of IFN-γ, IL-4 and IL-13 were measured by ELISA kits (IFN-γ: Quantikine Human IFN-γ, R&D Systems – lower limit of detection: 8.0 pg/mL; IL-4: Quantikine Human HS IL-4, R&S Systems – lower limit of detection: 0.11 pg/mL; IL-13: Human IL-13 ELISA, Bender MedSystems – lower limit of detection: 0.7 pg/mL). The present study was approved by the institutional ethical committee (reception number: 24, approved by institutional ethical committee of University of the Ryukyus on July 1st, 2008). Written informed consent was obtained from all participants.
Statistical analysis
Differences between groups were analyzed by Mann Whitney non-parametric U-test. Correlations were analyzed by Spearman’s rank test. P values were considered as significant when < 0.05.
| Results | ▴Top |
Serum levels of BAFF/APRIL in patients with Graves’ diseases
Serum BAFF level was significantly elevated in patients with GD compared to HD (1329 ± 435 pg/mL vs. 983 ± 308 pg/mL, P < 0.01) (Fig. 1A). On the other hand, serum APRIL level showed no difference between patients with GD and HD (6121 ± 4181 pg/mL vs. 6343 ± 4694 pg/mL) (Fig. 1A). Levels of serum cytokines associated with helper T1 or T2 cells, IFN-γ, IL-4 and IL-13, were measured in patients with GD and HD. Serum level of IFN-γ showed no difference between patients with GD and HD (Fig. 1B). Similarly, serum levels of IL-4 and IL-13 did not show any difference between two groups (Fig. 1B).
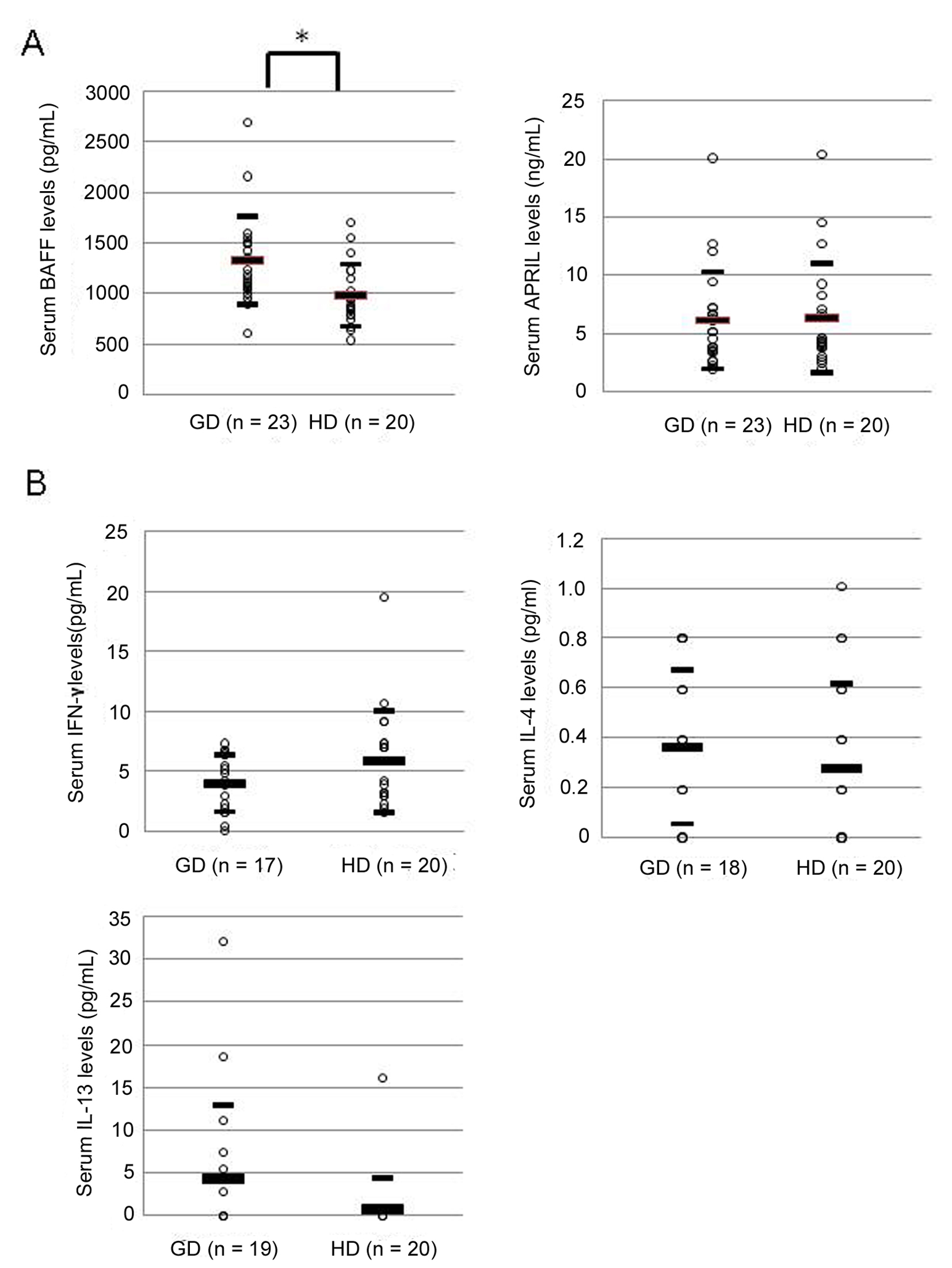 Click for large image | Figure 1. Serum levels of BAFF, APRIL (A), and IFN-γ, IL-4, and IL-13 (B) in patients with Graves’ disease. GD: patients with Graves’ disease; HD: healthy donors. Large bar shows an average level, and small bars show ± standard deviation. Data were analyzed by Mann Whitney non-parametric U-test. * P < 0.01 |
Relation between serum BAFF level and the activity of Graves’ diseases
Potential relationship between serum BAFF level and the activity of GD was examined. It is widely accepted that several symptoms of thyrotoxicosis are correlated to serum levels of T4 or FT4 [17]. Furthermore, high level of FT3 in patients with GD is correlated with the progression of GD [17]. Thus, we compared the serum levels of BAFF and thyroid hormones including FT3, FT4, and TRAb. Serum BAFF level was not correlated with FT3, FT4 and TRAb levels (Fig. 2). Notably, however, serum BAFF level was significantly correlated with FT3 to FT4 ratio (FT3/FT4), a useful indicator of the activity in GD (correlation coefficient: 0.477, P < 0.03) (Fig. 2).
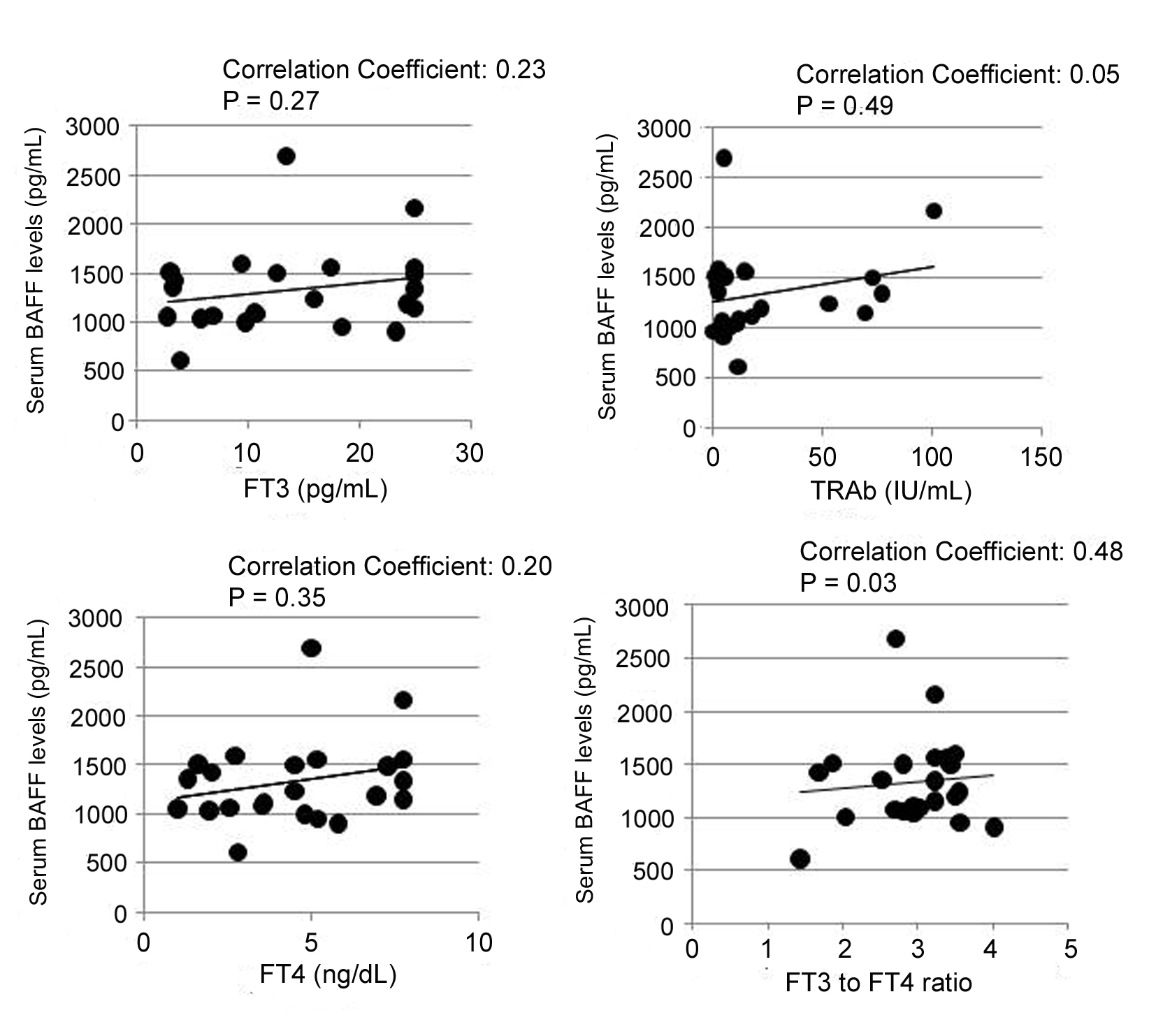 Click for large image | Figure 2. Positive correlations between serum BAFF level and thyroidal parameters. GD: patients with Graves’ disease; HD: healthy donors; FT3: free triiodothyronine; FT4: free thyroxine; TRAb: TSH receptor autoantibody. Vertical axis shows serum BAFF levels, and straight axis shows each thyroidal parameters (FT3, FT4, TRAb, and FT3 to FT4 ratio (FT3/FT4)). Data were analyzed by Spearman’s correlation coefficient by rank test. |
| Discussion | ▴Top |
The major finding of the present study is that serum BAFF level is significantly elevated in patients with GD patients, and the value is significantly correlated with FT3 to FT4 ratio, which is a widely accepted clinical indicator of GD activity. Previously, Fabris et al. [14] showed that serum BAFF level was elevated in patients with GD and Hashimoto’s thyroiditis. However, the report did not address the implication of circulating BAFF level in the disease activities of autoimmune thyroid diseases. In the present study, we demonstrated for the first time that serum BAFF level was correlated with the ratio of FT3 to FT4 ratio (FT3/FT4). Although sample numbers studied were limited, this result raises a possibility that serum BAFF level in patients with GD is a reflection of the disease activity. Mechanisms whereby serum BAFF level is elevated are supposed to be related with the increased production of BAFF by myeloid cells, increased release of BAFF from several kinds of cells, and decreased degradation of BAFF from blood [2, 3]. In this context, further studies are warranted to determine whether BAFF is overproduced in thyroid tissue in patients with GD.
Previous studies also showed that serum level of soluble IL-2 receptor in patients with GD was elevated, accompanied by an increase in circulating level of thyroid hormones (T3, T4, FT3, FT4) [18]. Moreover, in patients with GD, level of serum soluble IL-2 was correlated with those of thyroid hormones [18]. Taken together, it is reasonable to speculate that release of some types of cytokines is increased in hyperthyroid state. Elevated level of serum BAFF may also be a facet of hyperthyroid state.
It is widely accepted that the T3 to T4 ratio (T3/T4) was a simple and helpful clinical index for discrimination of the two types of thyrotoxicosis, GD and destruction-induced thyrotoxicosis [19, 20]. Only GD patients show a trend to have higher level of T3/T4 [19, 20]. On the other hand, destruction-induced thyrotoxicosis is a simple hyperthyroidism and does not go through the immune mechanism. Thus, it is likely that higher level of FT3/FT4 is an index of immunological activity of GD.
In the present study, serum level of APRIL was not changed in patients with GD. It is interesting to note that receptors for BAFF and APRIL are overlapped, but not completely the same. Both BAFF and APRIL bind to B-cell maturation antigen (BCMA), and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) [21]. Notably, BAFF binds specifically to one receptor called BAFF receptor (BAFF-R), and APRIL has another distinct receptor called heparin sulfate proteoglycans (HSPG) [4]. Therefore, the difference in the type of receptors may be relevant to the pathophysiology of GD. Further studies are warranted to clarify the clinical implication of circulating APRIL in GD.
Excessive differentiation and activation of B lymphocytes result in hypergammaglobulinemia and elevation in the circulating level of TSAb. However, the present study did not show the correlation between serum BAFF level and TRAb. It should be noted that our study did not directly measure the TSAb. TBII included TSAb and thyroid stimulation blocking antibody (TSBAb). A recent study showed that the severity of GD did not necessarily correlate with the level of circulating TRAb [1]. In this context, further studies are required to see if levels of other autoantibodies may correlate with serum BAFF levels.
We preliminarily examined serum BAFF levels in seven patients after the treatment with oral anti-thyroid drugs for 6 - 24 months. Noteworthy is that serum BAFF levels showed an apparent trend to decrease compared to the initial value (initial value: 1333 ± 661 pg/mL, value after the treatment: 1107 ± 277 pg/mL), but there was no correlation between serum BAFF levels and a line of thyroidal parameters (TSH, FT3, FT4, TRAb) (data not shown).
Recent animal study demonstrated that the blockade of BAFF or BAFF+APRIL with BAFF-specific receptor-Fc and B cell maturation antigen-Fc resulted in marked improvement of the induced hyperthyroidism [22]. Another study showed that the transplantation of intrathyroidal lymphocytes in human patients of GD with high TSAb levels to severe combined immunodeficient mice (SCID mice) resulted in elevating human immunoglobulin G and M. Taken together, therapeutic implication of BAFF/APRIL for GD may open a fresh avenue in the pathophysiology of autoimmune thyroid diseases.
In summary, the present study demonstrates for the first time that serum BAFF level was correlated with the ratio of FT3/FT4, an indicator of the disease activity in GD, thereby offering a novel opportunity to evaluate GD.
Grant
This work was supported in part by Special Account Budget for Education and Research, granted by the Japan Ministry of Education (2011), the Takeda Science Foundation (Specified Research Grant) (2010), Grants-in-Aid (MEXT, JAPAN) (Category C) (No. 22591014, 22592105), Okinawa Medical Science Research Foundation (Research promotion) (2011), and the University of the Ryukyus Foundation (2010).
| References | ▴Top |
- Hadj-Kacem H, Rebuffat S, Mnif-Feki M, Belguith-Maalej S, Ayadi H, Peraldi-Roux S. Autoimmune thyroid diseases: genetic susceptibility of thyroid-specific genes and thyroid autoantigens contributions. Int J Immunogenet 2009;36(2):85-96.
pubmed doi - Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 1999;189(11):1747-1756.
pubmed doi - Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999;285(5425):260-263.
pubmed doi - Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol 2007;19(3):327-336.
pubmed doi - Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999;190(11):1697-1710.
pubmed doi - Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 2001;44(6):1313-1319.
pubmed doi - Zhang J, Roschke V, Baker KP, Wang Z, Alarcon GS, Fessler BJ, Bastian H, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001;166(1):6-10.
pubmed - Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, Sellam J, et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res Ther 2006;8(2):R51.
pubmed doi - Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med 1998;188(6):1185-1190.
pubmed doi - Mukhopadhyay A, Ni J, Zhai Y, Yu GL, Aggarwal BB. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J Biol Chem 1999;274(23):15978-15981.
pubmed doi - Morel J, Roubille C, Planelles L, Rocha C, Fernandez L, Lukas C, Hahne M, et al. Serum levels of tumour necrosis factor family members a proliferation-inducing ligand (APRIL) and B lymphocyte stimulator (BLyS) are inversely correlated in systemic lupus erythematosus. Ann Rheum Dis 2009;68(6):997-1002.
pubmed doi - Gu D, Ge J, Du W, Xue F, Chen Z, Zhao H, Zhou Z, et al. Raised expression of APRIL in Chinese patients with immune thrombocytopenia and its clinical implications. Autoimmunity 2009;42(8):692-698.
pubmed doi - Stohl W. SLE—systemic lupus erythematosus: a BLySful, yet BAFFling, disorder. Arthritis Res Ther 2003;5(3):136-138.
pubmed doi - Fabris M, Grimaldi F, Villalta D, Picierno A, Fabro C, Bolzan M, De Vita S, et al. BLyS and April serum levels in patients with autoimmune thyroid diseases. Autoimmun Rev 2010;9(3):165-169.
pubmed doi - Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto's thyroiditis (Th1) and Graves' disease (Th2). Neuroimmunomodulation 2004;11(4):209-213.
pubmed doi - Simmonds MJ, Gough SC. Unravelling the genetic complexity of autoimmune thyroid disease: HLA, CTLA-4 and beyond. Clin Exp Immunol 2004;136(1):1-10.
pubmed doi - Yoshimura Noh J, Momotani N, Fukada S, Ito K, Miyauchi A, Amino N. Ratio of serum free triiodothyronine to free thyroxine in Graves' hyperthyroidism and thyrotoxicosis caused by painless thyroiditis. Endocr J 2005;52(5):537-542.
pubmed doi - Koukkou E, Panayiotidis P, Alevizou-Terzaki V, Thalassinos N. High levels of serum soluble interleukin-2 receptors in hyperthyroid patients: correlation with serum thyroid hormones and independence from the etiology of the hyperthyroidism. J Clin Endocrinol Metab 1991;73(4):771-776.
pubmed doi - Amino N, Yabu Y, Miki T, Morimoto S, Kumahara Y, Mori H, Iwatani Y, et al. Serum ratio of triiodothyronine to thyroxine, and thyroxine-binding globulin and calcitonin concentrations in Graves' disease and destruction-induced thyrotoxicosis. J Clin Endocrinol Metab 1981;53(1):113-116.
pubmed doi - Amino N, Yabu Y, Miyai K, Fujie T, Azukizawa M, Onishi T, Kumahara Y. Differentiation of thyrotoxicosis induced by thyroid destruction from Graves' disease. Lancet 1978;2(8085):344-346.
pubmed doi - Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol 2002;2(7):465-475.
pubmed doi - Gilbert JA, Kalled SL, Moorhead J, Hess DM, Rennert P, Li Z, Khan MZ, et al. Treatment of autoimmune hyperthyroidism in a murine model of Graves' disease with tumor necrosis factor-family ligand inhibitors suggests a key role for B cell activating factor in disease pathology. Endocrinology 2006;147(10):4561-4568.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
