| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 5, Number 1-2, April 2015, pages 192-195
Treatment of Thyroxine Malabsorption
Jeremiah Kempkea, Hammad Hussainb, Bhavika Bhanc, Leland Gravesd, e
aDepartment of Endocrinology, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS 66160, USA
bDepartment of Endocrinology, Quincy Medical Group, 1025 Maine St, Quincy, IL 62301, USA
cDepartment of Endocrinology, St. Luke’s Health System, 12330 Metcalf Ave Suite 500A, Overland Park, KS 66213, USA
dDivision of Metabolism, Endocrinology, and Genetics, University of Kansas Medical Center, MS 2024, 3901 Rainbow Blvd, Kansas City, KS 66160, USA
eCorresponding Author: Leland Graves, Division of Metabolism, Endocrinology, and Genetics, University of Kansas Medical Center, MS 2024, 3901 Rainbow Blvd, Kansas City, KS 66160, USA
Manuscript accepted for publication April 09, 2015
Short title: Thyroxine Malabsorption
doi: http://dx.doi.org/10.14740/jem277w
| Abstract | ▴Top |
The aim of the case study was to report the successful use of intramuscular levothyroxine (L-T4) in two patients with profound oral L-T4 malabsorption. We present two cases of patients who remained hypothyroid despite very high oral doses of L-T4. In both cases, poor L-T4 absorption was documented with an absorption study. Intramuscular L-T4 injections were initiated in both cases and doses were titrated to achieve normalization of thyroid stimulating hormone (TSH) and free thyroxine (free T4). Additionally, after achieving euthyroidism for a period of time, each patient was able to successfully transit back to oral administration of L-T4. Synthetic oral L-T4 is the drug of choice for replacement therapy in patients with hypothyroidism. Whenever euthyroidism cannot be achieved despite escalating L-T4 doses, the presence of interfering factors must be considered. These factors include low patient compliance or reduced L-T4 absorption due to other dietary factors, concomitant medication use or gastrointestinal disease. Furthermore, severe hypothyroidism itself may impair absorption, presumably due to edema of the small bowel mucosa. Parenteral L-T4 has been shown to be an effective method of replacing thyroid hormone in those patients with apparent thyroid hormone malabsorption. However, there is no consensus or guidelines available to aid physicians in the use of parenteral L-T4. In patients with hypothyroidism despite large doses of levothyroxine, physicians should consider thyroid malabsorption once compliance and interfering medicines have been ruled out. In these patients, if no correctable interfering factor is identified, intramuscular replacement appears to be an effective alternative.
Keywords: Levothyroxine; Malabsorption; Hypothyroidism
| Introduction | ▴Top |
Hypothyroidism is a common endocrine disorder and in most cases in iodine sufficient areas is a primary autoimmune process in which the thyroid gland produces insufficient amounts of thyroid hormone. Another common cause is surgical removal of the thyroid, which may be done for a variety of reasons. Synthetic levothyroxine (L-T4) is the drug of choice for replacement therapy in patients with hypothyroidism. Although the average dose for effective and optimal replacement of L-T4 varies somewhat from patient to patient, most hypothyroid patients are managed within a fairly narrow dose window that varies according to body weight, the average being near 1.6 - 1.8 μg/kg [1]. Effectiveness of a dose of L-T4 is determined by measuring thyroid stimulating hormone (TSH) and free thyroxine (free T4).
In some cases, L-T4 doses exceeding the expected calculated daily requirements are needed to achieve the therapeutic goal or the goal cannot be achieved despite escalating doses. In these cases, several factors must be considered. These factors include low patient compliance, reduced L-T4 absorption from concomitant interfering dietary factors or use of interfering medications, or gastrointestinal disorders contributing to malabsorption. Furthermore, severe hypothyroidism itself may impair absorption, presumably due to edema of the small bowel mucosa. We report two such cases of profound L-T4 malabsorption that responded well to intramuscular L-T4 replacement.
| Case Reports | ▴Top |
Case 1
A 35-year-old female with Crohn’s disease underwent thyroidectomy for multinodular goiter. Surgical pathology was benign and L-T4 replacement was started at 1.6 μg/kg/day and escalated to a dose as high as 1,500 μg daily (approximately 24 μg/kg/day) with persistent biochemical and clinical hypothyroidism. Appropriate adherence to oral administration and avoidance of interfering medications was confirmed. An oral L-T4 absorption study was performed using 1 mg of oral L-T4 which was chewed and swallowed by the patient while being observed. Subsequent total thyroxine levels were obtained at 60, 120, 180, and 240 min (Fig. 1) [2]. This revealed L-T4 absorption of approximately 13% of normal control subjects [3].
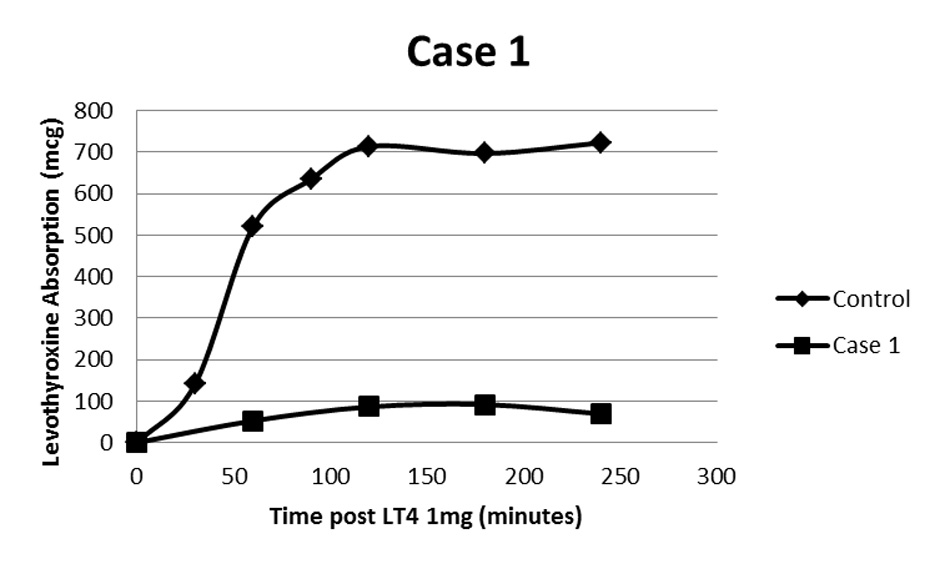 Click for large image | Figure 1. Oral levothyroxine absorption study following 1 mg L-T4. Case 1 demonstrates a maximum of about 13% absorption of control [2]. |
Intramuscular L-T4 (lyophilized levothyroxine sodium 200 μg, reconstituted with 5 mL of 0.9% sodium chloride; Fresenius Kabi USA, LLC, Lake Zurich, IL) was started with an initial dose of 0.3 μg/kg/day divided into twice weekly injections (i.e. for her weight of 61 kg a total of 128 μg per week divided into 64 μg twice weekly given as 1.6 mL of 200 μg lyophilized levothyroxine reconstituted in 5 mL of saline). This was increased to a final dose of 200 μg intramuscularly twice weekly or approximately 0.9 μg/kg/day, with normalization of thyroid labs and resolution of clinical hypothyroidism. There were no adverse effects of intramuscular injections recognized. After 2 years, the patient was able to be transitioned back to oral L-T4. She did require a higher than expected oral dose, 250 μg or about 5 μg/kg daily to achieve a normal TSH which is likely due to underlying Crohn’s disease.
Case 2
A 43-year-old female with primary hypothyroidism was initially treated with oral L-T4 with doses escalating to 1,500 μg daily (approximately 17 μg/kg/day). She required admission to the hospital with profound hypothyroidism including pericardial effusion and mental status changes and was treated with intravenous L-T4 acutely. An oral L-T4 absorption study was performed in the same manner as case 1 (Fig. 2) [2] which revealed levothyroxine absorption to be approximately 7% of controls [3].
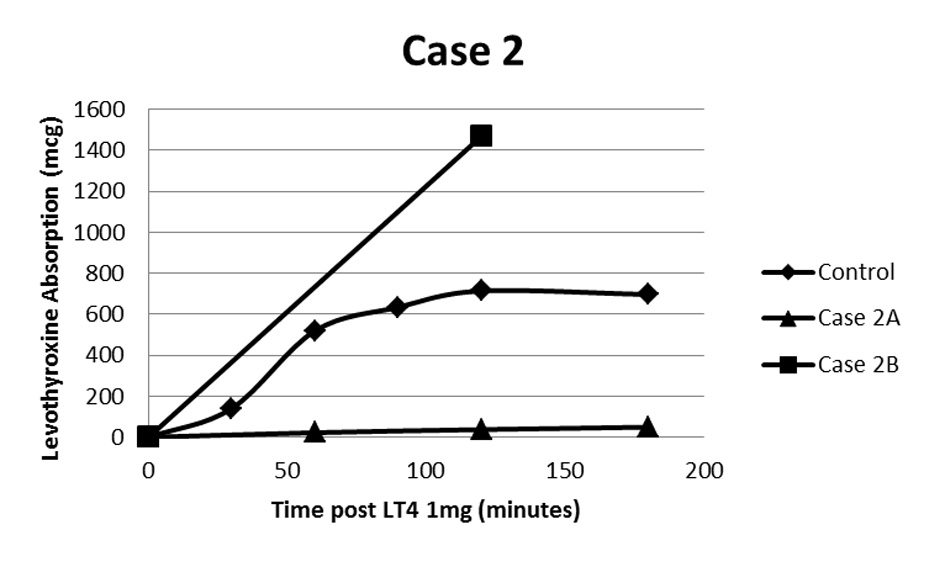 Click for large image | Figure 2. Oral L-T4 absorption study following 1mg L-T4. Case 2A demonstrates a maximum of about 7% absorption compared to control [2]. Case 2B demonstrates above normal absorption once patient had achieved euthyroidism using intramuscular L-T4 and was subsequently transitioned back to oral L-T4. The calculated supranormal absorption is likely related to inaccuracy of the estimated volume of distribution at high BMI. |
Intramuscular L-T4 was started at a dose of 0.3 μg/kg/day divided into two injections weekly. This was titrated to a dose of 1.0 μg/kg/day given as 200 μg three times weekly. This resulted in normalization of thyroid studies and resolution of hypothyroid symptoms. After almost 5 years, she was able to be transitioned back to oral L-T4. Her current dose after adjustment is 100 μg 6 days weekly, which is approximately 1.0 μg/kg. This patient also underwent an abbreviated L-T4 absorption study (only baseline and 2 h levels checked) following return to successful oral replacement which actually demonstrated supra-normal absorption of 1 mg L-T4 (Fig. 2). This likely represents an artifact of inaccuracy of the estimated volume of distribution of L-T4 at high BMI. Regardless, absorption of 1 mg L-T4 was clearly increased when compared to her study done prior to achieving euthyroidism with intramuscular L-T4.
| Discussion | ▴Top |
Approximately 62-82% of L-T4 is absorbed after oral administration. Most of the absorption occurs within the first 3 h of ingestion and is localized mainly in the jejunum and ileum [4]. The absorption occurs best on an empty stomach which may reflect the importance of gastric acidity in this process [5]. Absorption of L-T4 has been shown to be reduced by certain foods such as fiber [6], coffee [7], and soy protein [8, 9]. Gastrointestinal disorders such as lactose intolerance [10] and celiac disease [11, 12] inflammatory bowel disease and other malabsorptive disorders may result in L-T4 malabsorption. Medications such as sucralfate [3], ferrous sulfate [13, 14], calcium carbonate [15, 16], and proton pump inhibitors [17-19] are also known to affect absorption of L-T4. Altered absorption generally manifests as abnormal thyroid function testing (TSH and free T4) and an apparent under dosing of medication, which may be intermittent or persistent depending on the exact interfering factor. Alternatively, another common reason for failure to normalize TSH with appropriate dosing of L-T4 is a lack of regular compliance with medication [20].
Conversely, there have been previously described ultimately unresolved cases of hypothyroidism refractory to oral therapy where no interfering factors could be found [21-24]. Almost all have been described in women, mostly aged 40 - 50 and with history of papillary thyroid cancer and surgical hypothyroidism [21-23]. It is also noteworthy that in a majority of these cases, absorption capacity improved once stable euthyroidism had been achieved by intermittent parenteral levothyroxine administration. This supports the hypothesis of intestinal mucosal edema with severe hypothyroidism affecting L-T4 absorption. However in one of the reported cases [23], tissue micro-architecture between small bowel biopsies obtained at the time of severe hypothyroidism and euthyroidism was not different. The authors in this case proposed a specific enteral defect. Similarly, our two cases both had improved oral absorption of L-T4 after achieving euthyroidism with intramuscular administration for a period of time and one patient subsequently had an absorption study demonstrating normal absorption.
We have reported two cases where patients could not achieve euthyroidism despite of escalating doses of oral L-T4. There were no identified interfering medications or correctable conditions including poor compliance. These patients both had documented L-T4 absorption well below normal range on kinetic absorption testing. In both cases, intramuscular L-T4 administration resulted in normalization of thyroid function testing and clinical findings of hypothyroidism. Additionally, after achieving euthyroidism for 2 - 5 years, both patients were able to transit to oral replacement and maintain euthyroidism at L-T4 doses that did not correct hypothyroidism prior to intramuscular treatment. One patient (case 2) also had demonstrable improvement on L-T4 absorption testing. These findings support the theory of intestinal mucosal alteration due to severe hypothyroidism that may be correctable with restoration of euthyroidism using parenteral L-T4.
Currently, no guidelines or consensus exist to aid providers in using intramuscular L-T4 replacement. There are however some general considerations that apply. First, parenteral L-T4 is considered roughly twice as potent as oral replacement, which generally requires 1.6 - 1.8 g/kg/day to achieve euthyroidism [1]. Second, the half-life of L-T4 is 6 - 7 days and can be longer in a hypothyroid patient, so daily injections are not needed. To this point, a kinetic mathematical model produced by Hays predicted that serum thyroxine levels would be normal with both weekly and biweekly intramuscular L-T4 dosing, although there was more fluctuation in levels with less frequent dosing [25]. Therefore, a reasonable starting dose would be 0.8 μg/kg/day divided into one to three weekly injections. In our two cases, we opted for a more conservative starting dose of 0.3 μg/kg/day divided into two weekly doses with gradual dose titration based on thyroid function testing while monitoring for common side effects of hyperthyroidism. In our cases, the final doses required for euthyroidism were 1.0 μg/kg/day and 1.2 μg/kg/day. No side effects of injections were noted and no signs or symptoms of hyperthyroidism were identified.
Conclusion
Levothyroxine is generally recognized to be a medication with a relatively narrow toxic to therapeutic ratio [2]. If the goal of therapy is maintenance of a specific serum TSH within a relatively narrow range, without significant oscillations, then special attention should be paid to concurrent medications or addition of new medications, timing of L-T4 ingestion and dietary modifications. Maintaining specific TSH targets are particularly important in patients who are pregnant, elderly, or have diagnoses of thyroid cancer, cardiac disease, or osteoporosis. Avoidance of subclinical thyroid disease may be particularly critical in these populations [26].
Intramuscular L-T4 can be a safe and effective method of replacing thyroid hormone in patients with unexplained or unresolved thyroid hormone malabsorption as evidenced by persistent hypothyroidism despite escalating doses of oral L-T4 with no identified underlying reason for malabsorption that is correctable and no compliance problems. In these cases, we suggest initiating intramuscular L-T4 at a conservative dose and titrating as necessary based on thyroid functions testing while monitoring for signs and symptoms of hyperthyroidism. We have used a starting dose of 0.3 μg/kg/day divided in two to three weekly doses, but slightly higher doses are likely reasonable as well. These patients may eventually be able to successfully transit back to oral replacement; however, the time frame when this might be possible is not clear.
Disclosure
The authors have no financial or other conflicts of interest to disclose.
| References | ▴Top |
- Mandel SJ, Brent GA, Larsen PR. Levothyroxine therapy in patients with thyroid disease. Ann Intern Med. 1993;119(6):492-502.
doi pubmed - Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf). 1988;28(3):325-333.
doi - Sherman SI, Tielens ET, Ladenson PW. Sucralfate causes malabsorption of L-thyroxine. Am J Med. 1994;96(6):531-535.
doi - Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F. Delayed intestinal absorption of levothyroxine. Thyroid. 1995;5(4):249-253.
doi pubmed - Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94(10):3905-3912.
doi pubmed - Liel Y, Harman-Boehm I, Shany S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab. 1996;81(2):857-859.
doi - Benvenga S, Bartolone L, Pappalardo MA, Russo A, Lapa D, Giorgianni G, Saraceno G, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18(3):293-301.
doi pubmed - Bell DS, Ovalle F. Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract. 2001;7(3):193-194.
doi pubmed - Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16(3):249-258.
doi pubmed - Munoz-Torres M, Varsavsky M, Alonso G. Lactose intolerance revealed by severe resistance to treatment with levothyroxine. Thyroid. 2006;16(11):1171-1173.
doi pubmed - d'Esteve-Bonetti L, Bennet AP, Malet D, Hoff M, Louvet JP, Caron P. Gluten-induced enteropathy (coeliac disease) revealed by resistance to treatment with levothyroxine and alfacalcidol in a sixty-eight-year-old patient: a case report. Thyroid. 2002;12(7):633-636.
doi pubmed - McDermott JH, Coss A, Walsh CH. Celiac disease presenting as resistant hypothyroidism. Thyroid. 2005;15(4):386-388.
doi pubmed - Campbell NR, Hasinoff BB, Stalts H, Rao B, Wong NC. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117(12):1010-1013.
doi pubmed - Shakir KM, Chute JP, Aprill BS, Lazarus AA. Ferrous sulfate-induced increase in requirement for thyroxine in a patient with primary hypothyroidism. South Med J. 1997;90(6):637-639.
doi pubmed - Singh N, Weisler SL, Hershman JM. The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid. 2001;11(10):967-971.
doi pubmed - Csako G, McGriff NJ, Rotman-Pikielny P, Sarlis NJ, Pucino F. Exaggerated levothyroxine malabsorption due to calcium carbonate supplementation in gastrointestinal disorders. Ann Pharmacother. 2001;35(12):1578-1583.
doi pubmed - Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, Kim PJ. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13(4):345-349.
doi pubmed - Dietrich JW, Gieselbrecht K, Holl RW, Boehm BO. Absorption kinetics of levothyroxine is not altered by proton-pump inhibitor therapy. Horm Metab Res. 2006;38(1):57-59.
doi pubmed - Ananthakrishnan S, Braverman LE, Levin RM, Magnani B, Pearce EN. The effect of famotidine, esomeprazole, and ezetimibe on levothyroxine absorption. Thyroid. 2008;18(5):493-498.
doi pubmed - England ML, Hershman JM. Serum TSH concentration as an aid to monitoring compliance with thyroid hormone therapy in hypothyroidism. Am J Med Sci. 1986;292(5):264-266.
doi - Jauk B, Mikosch P, Gallowitsch HJ, Kresnik E, Molnar M, Gomez I, Lind P. Unusual malabsorption of levothyroxine. Thyroid. 2000;10(1):93-95.
doi pubmed - Nagaoka T, Miyakoshi H, Takamura T, Nagai Y, Matsushita S, Kaneko S, Kobayashi K. A case of refractory hypothyroidism requiring daily intravenous thyroxine. J Int Med Res. 2002;30(4):463-465.
doi pubmed - Tonjes A, Karger S, Koch CA, Paschke R, Tannapfel A, Stumvoll M, Fuhrer D. Impaired enteral levothyroxine absorption in hypothyroidism refractory to oral therapy after thyroid ablation for papillary thyroid cancer: case report and kinetic studies. Thyroid. 2006;16(10):1047-1051.
doi pubmed - Anderson L, Joseph F, Goenka N, Patel V. Isolated thyroxine malabsorption treated with intramuscular thyroxine injections. Am J Med Sci. 2009;337(2):150-152.
doi pubmed - Hays MT. Parenteral thyroxine administration. Thyroid. 2007;17(2):127-129.
doi pubmed - Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
