| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 9, Number 3, June 2019, pages 51-62
Efficacy and Safety of Ipragliflozin in Patients With Type 2 Diabetes: ASSIGN-K Study
Kotaro Iemitsua, Takehiro Kawataa, Takashi Iizukaa, Masahiro Takihataa, Masahiko Takaia, Shigeru Nakajimaa, Nobuaki Minamia, Shinichi Umezawaa, Akira Kanamoria, Hiroshi Takedaa, Shogo Itoa, Taisuke Kikuchia, Hikaru Amemiyaa, Mizuki Kaneshiroa, Atsuko Mokuboa, Tetsuo Takumaa, Hideo Machimuraa, Keiji Tanakaa, Taro Asakuraa, Akira Kubotaa, Sachio Aoyagia, Kazuhiko Hoshinoa, Masashi Ishikawaa, Yoko Matsuzawaa, Mitsuo Obanaa, Nobuo Sasaia, Hideaki Kaneshigea, Fuyuki Minagawaa, Tatsuya Saitoa, Kazuaki Shinodaa, Masaaki Miyakawaa, Yasushi Tanakab, Yasuo Terauchic, Ikuro Matsubaa, d
aKanagawa Physicians Association, Kanagawa, Japan
bDivision of Metabolism and Endocrinology, Department of Internal Medicine, St. Marianna University School of Medicine, Kanagawa, Japan
cDepartment of Endocrinology and Metabolism, Yokohama City University, Kanagawa, Japan
dCorresponding Author: Ikuro Matsuba, Matsuba Medical Clinic, 2-159 Tsukagoshi, Saiwai-ku, Kawasaki, Kanagawa 212-0024, Japan
Manuscript submitted April 23, 2019, accepted May 16, 2019
Short title: Ipragliflozin for Type 2 Diabetes
doi: https://doi.org/10.14740/jem570
| Abstract | ▴Top |
Background: Sodium glucose cotransporter 2 (SGLT2) inhibitors achieve good glycemic control in patients with type 2 diabetes, as well as reducing body weight and blood pressure. However, no large-scale clinical studies of SGLT2 inhibitors have been performed in Japan. The ASSIGN-K study investigated the efficacy and safety of ipragliflozin, a selective SGLT2 inhibitor, in routine clinical practice.
Methods: ASSIGN-K was an investigator-initiated, multicenter, prospective observational study of ipragliflozin that enrolled Japanese patients with type 2 diabetes who showed inadequate glycemic control despite diet and exercise with/without medication. Patients received ipragliflozin (50 mg/day) as monotherapy or combined with other antidiabetic agents for up to 104 weeks.
Results: In 301 patients who completed104 weeks of ipragliflozin treatment, hemoglobin A1c was significantly reduced from 8.07% at baseline to 7.24% (P < 0.001), while fasting blood glucose (n = 86) and postprandial blood glucose (n = 75) were decreased significantly by 19.8 mg/dL (P < 0.001) and 29.6 mg/dL (P < 0.05), respectively. Body weight (n = 217) also showed a significant decrease from 79.1 kg to 76.2 kg (P < 0.001). In addition, body fat (n = 217) and fat-free mass (n = 217) were significantly reduced by 1.87 kg and 1.02 kg, respectively (both P < 0.001), while total body water displayed a significant decrease of 0.74 kg (P < 0.001). Similar results were obtained when 30 patients aged ≥ 65 years were compared with 187 patients aged < 65 years. Multiple regression analysis revealed greater improvement of hemoglobin A1c when the baseline value was higher, while improvement became less marked as the duration of diabetes became longer and as the baseline body mass index increased. In patients achieving hemoglobin A1c < 7.0%, baseline hemoglobin A1c was significantly lower than in patients not achieving hemoglobin A1c < 7.0%, (7.45% vs. 8.54%, P < 0.001) and the duration of diabetes was significantly shorter (8.66 vs. 10.69 years, P = 0.002). Ketoacidosis was only reported in patients who continued ipragliflozin treatment during intercurrent illness.
Conclusions: In patients with type 2 diabetes and inadequate glycemic control, ipragliflozin significantly improved hemoglobin A1c regardless of baseline characteristics, including age, sex, hemoglobin A1c, and body mass index. However, it is important to suspend treatment with ipragliflozin during intercurrent illness.
Keywords: Hemoglobin A1c; Ipragliflozin; Type 2 diabetes mellitus; Body mass index; Body weight; Waist circumference; Estimated glomerular filtration rate
| Introduction | ▴Top |
After glucose is filtered by the renal glomeruli, sodium glucose co-transporter 2 (SGLT2) plays a major role in its reabsorption from the proximal tubules [1, 2]. SGLT2 inhibitors improve hyperglycemia by blocking tubular glucose reabsorption. Japanese clinical studies have demonstrated that good glycemic control can be achieved with various SGLT2 inhibitors [3-9], and these drugs also reduce body weight and blood pressure [10]. Due to their unique mechanism of action, SGLT2 inhibitors can be combined with other antidiabetic agents, and are listed as an option for combination therapy in the 2015 update from the American Diabetes Association and the European Association for the Study of Diabetes [11]. Characteristic adverse reactions caused by SGLT2 inhibitors in clinical studies were reported to be urinary tract or genital infections, while serious reactions such as severe hypoglycemia, ketoacidosis, or generalized rash have occurred when these drugs were combined with insulin or sulfonylureas in the routine clinical setting. Dehydration due to osmotic diuresis associated with increased urinary glucose excretion has also been reported. Accordingly, the Japan Diabetes Society established recommendations for SGLT2 inhibitors in 2014 and 2016, including cautions about combined use with insulin or sulfonylureas, dehydration, urinary/genital tract infection in elderly patients, and suspension of use during intercurrent illness. Because SGLT2 inhibitors reduce body weight, these drugs are often used to treat patients with type 2 diabetes (T2D) who have insulin resistance and obesity. Large-scale clinical studies, including EMPA-REG and CANVAS, have demonstrated multiple actions of SGLT2 inhibitors in addition to lowering blood glucose, including cardioprotective and renoprotective effects, and the 2018 US Food and Drug Administration Guidelines state that these drugs reduce the risk of major atherosclerotic cardiovascular events, cardiovascular mortality, and progression of nephropathy. However, no large-scale clinical studies of SGLT2 inhibitors have been performed in Japanese patients. A phase II dose-finding study showed that ipragliflozin, a selective SGLT2 inhibitor, reduced hemoglobin A1c (HbA1c) and body weight in Japanese T2D patients [3]. In that study, the decrease of HbA1c was significantly larger in patients with lower baseline HbA1c levels, while other investigations of ipragliflozin have demonstrated a larger decrease of HbA1c in patients with high baseline HbA1c levels [4, 12-14].
ASSIGN-K was an investigator-initiated prospective multicenter study that examined the efficacy and safety of ipragliflozin in routine clinical practice. We have already reported data on glycemic control, safety, and the influence of patient characteristics on HbA1c after 12 and 24 weeks of treatment [15, 16]. This time, we examined the effects of ipragliflozin on HbA1c and body composition up to 104 weeks. Changes of HbA1c were assessed after stratification by various patient characteristics, and we also investigated differences between patients achieving/not achieving HbA1c < 7.0%.
| Materials and Methods | ▴Top |
Study outline
ASSIGN-K was an investigator-initiated multicenter prospective study that assessed the efficacy and safety of ipragliflozin as monotherapy (for patients who were treatment-naive or switched therapy) or combined with other antidiabetic agents in Japanese outpatients with T2D [15]. The study commenced in June 2014 at 33 participating clinics in Kanagawa Prefecture, Japan. After obtaining written informed consent, patients were registered by electronic data capture until November 31, 2017. This study was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Clinical Research. The study protocol and the informed consent document were approved by the institutional review boards of the participating hospitals before initiation of the investigation. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (no.: UMIN000014425).
Subjects
Japanese T2D patients aged at least 20 years at the time of giving informed consent were eligible if HbA1c (National Glycohemoglobin Standardization Program) was ≥ 6.0% and glycemic control was inadequate despite diet and exercise therapy with/without antidiabetic agents. Exclusion criteria were a history of hypersensitivity to ipragliflozin, a history of severe diabetic ketosis with coma or precoma, current severe infection, perioperative status, serious trauma, and severe renal dysfunction.
Study design
Subjects took ipragliflozin (50 mg/day) before or after breakfast for 104 weeks. Other SGLT2 inhibitors were prohibited, and diet/exercise therapy could not be initiated, discontinued, or reduced. If possible, there was also no initiation, discontinuation, or modification of antiplatelet agents, antihypertensive drugs, and lipid-lowering drugs.
Endpoints
The primary efficacy endpoint was the change of HbA1c from initiation of treatment (baseline) to week 104 of the study. Secondary efficacy endpoints were the changes of fasting blood glucose, postprandial blood glucose, body composition, weight, waist circumference, serum lipids, kidney function, and blood pressure from baseline to week 104, as well as the change of HbA1c stratified by baseline patient characteristics. These parameters were evaluated at baseline and in weeks 4, 12, 24, 36, 52, 78, and 104. Safety endpoints were the change of blood ketone bodies from baseline, adverse events (AEs), and adverse reactions (ARs). We also examined changes of liver function and mineral mass at 104 weeks. Patients who had completed 104 weeks of treatment by March 2016 were analyzed in this study.
Statistical analysis
Summary statistics or frequencies were calculated from actual data for patient characteristics, efficacy endpoints, and changes from baseline. Analysis of variance (ANOVA) was employed for stratified analysis of the change in each endpoint versus the change of HbA1c from baseline to week 104. Patient characteristics influencing the change of HbA1c were investigated by stepwise multiple regression analysis. For all analyses, significance was accepted at P < 0.05. To assess safety, the frequency and incidence of AEs were calculated. Preferred terms from MedDRA/J version 18.0 (Japanese Maintenance Organization, Tokyo, Japan) were used to list AEs and the system organ class was used to designate affected organs. AEs were classified as ARs if a causal relationship with ipragliflozin could not be excluded.
| Results | ▴Top |
Baseline characteristics of the patients
A total of 301 patients were eligible for efficacy analysis and 451 patients were enrolled in the safety analysis (Table 1). Most of the dropouts refused consent to participation in the second year of the study, with the main reason being considered to be a burdensome questionnaire. Baseline characteristics of the patients included in efficacy analysis are shown in Table 1. Their mean age was 55.5 ± 11.6 years, the male: female ratio was approximately 1:1, mean body mass index (BMI) was 29.4 ± 5.3 kg/m2, mean HbA1c was 8.01±1.43%, and average duration of diabetes was 9.7 ± 7.4 years. Concomitant conditions were dyslipidemia in 65.2%, hypertension in 57.4%, fatty liver in 48.3%, diabetic nephropathy in 29.0%, diabetic retinopathy in 13.7%, diabetic neuropathy in 13.3%, and macroangiopathy in 12.4%. At initiation of study treatment, 12.9% of patients received ipragliflozin monotherapy, while ipragliflozin was prescribed with one concomitant antidiabetic agent in 17.3% and with two or more concomitant antidiabetic agents in approximately 70%. Ipragliflozin was the first antidiabetic agent for 12.9% of the patients, while 8.6% were switched from other agents and ipragliflozin was added to current treatment in 78.5%. Concomitant drugs included dipeptidyl peptidase 4 inhibitors in 72.9% of the patients, biguanides in 66.1%, sulfonylureas in 36.2%, insulin in 22.3%, and thiazolidinediones in 14.7%.
 Click to view | Table 1. Baseline Characteristics of the Patients |
HbA1c, blood glucose, and weight
From baseline to week 104, fasting blood glucose (n = 86) showed a significant decrease by 19.8 mg/dL (from 153.5 to 133.7 mg/dL) and postprandial blood glucose (n = 75) was reduced by 29.6 mg/dL (from 188.9 to 159.3 mg/dL) (P < 0.001 and P < 0.05), respectively (Fig. 1). In addition, HbA1c (n = 301) was significantly decreased by 0.83% (from 8.07% to 7.24%, P < 0.001). Body weight (n = 217), BMI (n = 217), and waist circumference (n = 268) were significantly reduced by 2.9 kg (from 79.1 to 76.2 kg), 1.2 kg/m2 (from 29.4 to 28.2 kg/m2), and 2.9 cm (from 99.2 to 96.3 cm), respectively (all P < 0.001) (Fig. 2).
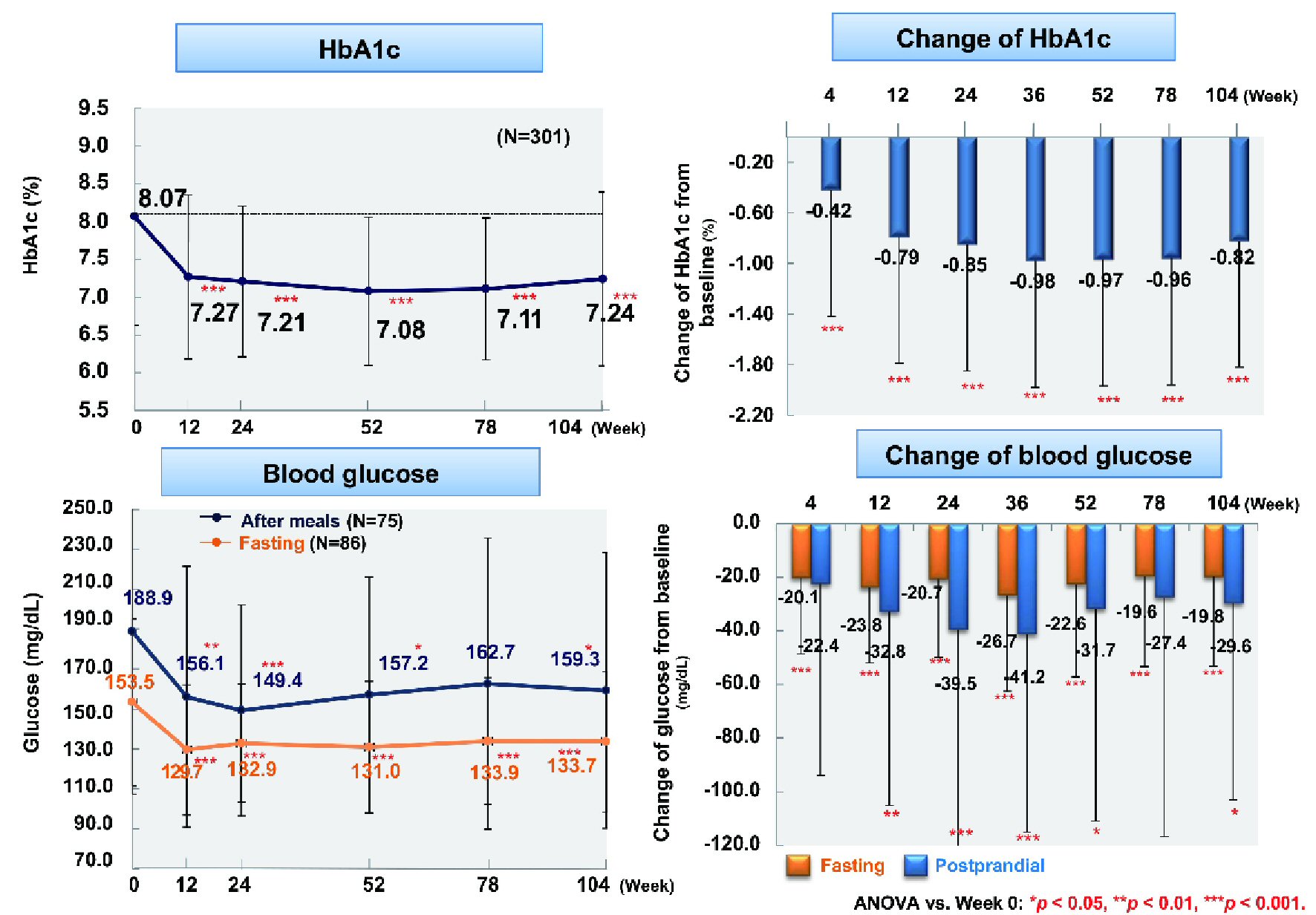 Click for large image | Figure 1. Changes of glycemic control. ANOVA: analysis of variance; HbA1c: hemoglobin A1c. |
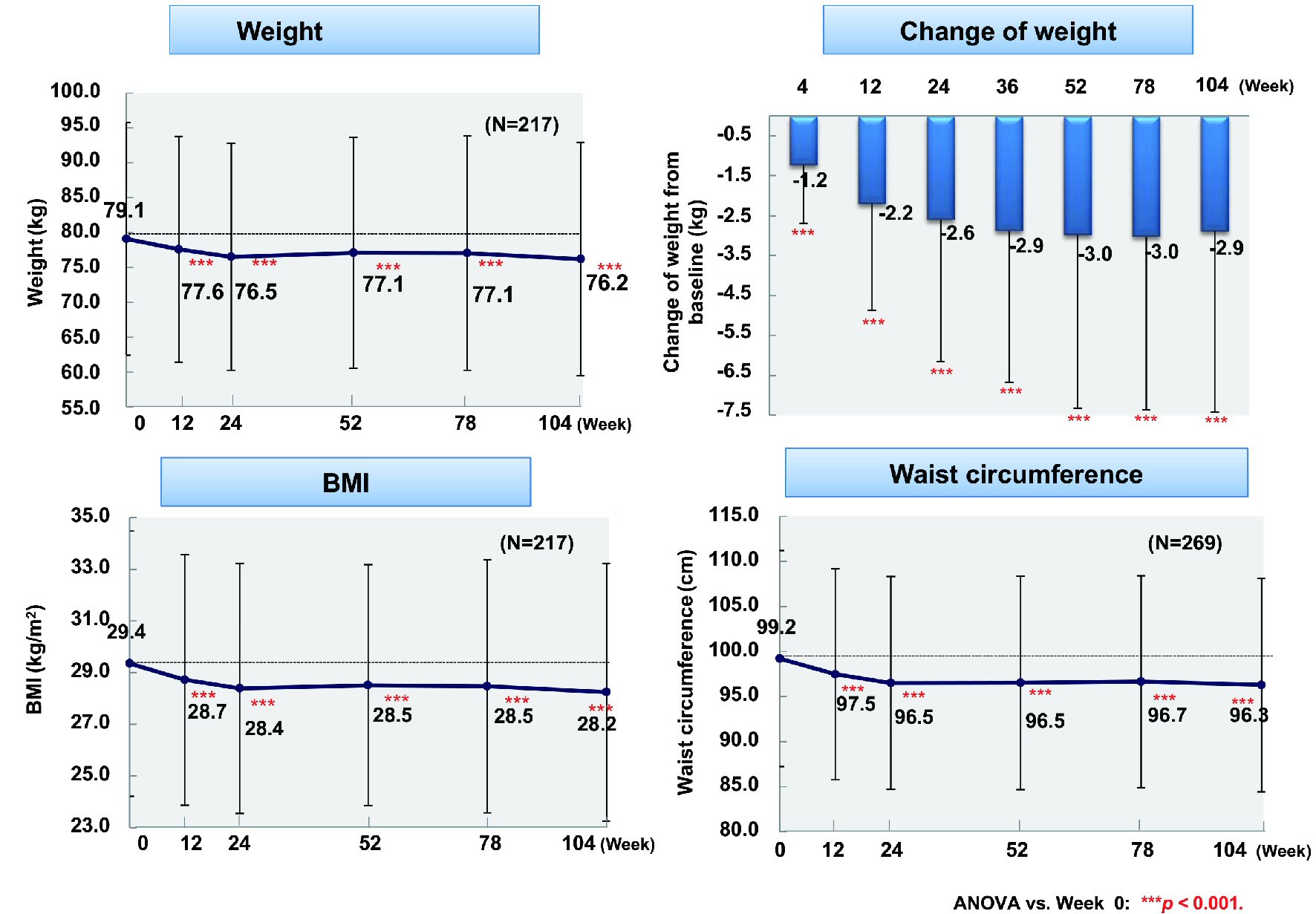 Click for large image | Figure 2. Changes of weight, BMI, and waist circumference. ANOVA: analysis of variance; BMI: body mass index. |
Changes of body composition
From baseline to week 104, body fat (n = 217) and fat-free mass (n = 217) showed a significant decrease by 1.87 kg and 1.02 kg, respectively (both P < 0.001) (Fig. 3). With regard to the fat-free mass components, muscle mass (n = 217) and mineral mass (n = 217) declined significantly by 0.87 kg and 0.15 kg (both P < 0.001). Total body water (n = 217) and protein (n = 217), which are the components of muscle mass, decreased by 0.74 kg (P < 0.001) and 0.13 kg (P < 0.05), respectively. Accordingly, weight loss was mainly due to reduction of body fat and total body water. Comparison between patients aged ≥ 65 years (n = 30) and patients aged < 65 years (n = 187) revealed body fat loss of 1.72 kg vs. 1.89 kg (P < 0.05 and P < 0.001, respectively), while the decrease of fat-free mass was 1.02 kg vs. 1.02 kg (n.s. and P < 0.001, respectively).
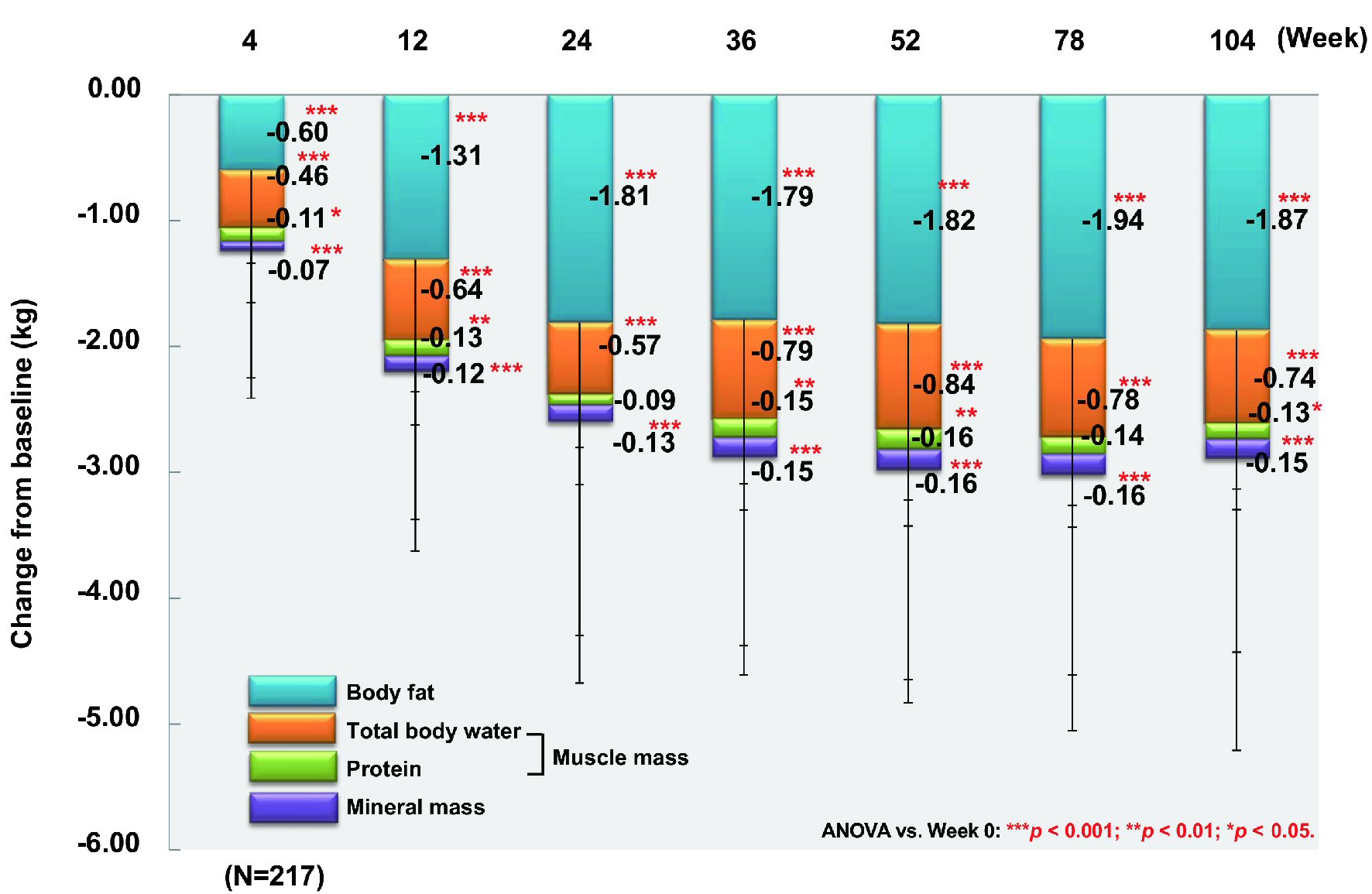 Click for large image | Figure 3. Changes of body fat, total body water, protein, and mineral mass. ANOVA: analysis of variance. |
Influence of patient characteristics
The change of HbA1c from baseline to week 104 was investigated in relation to age, sex, duration of diabetes, BMI, estimated glomerular filtration rate (eGFR), HbA1c, and ipragliflozin prescribing pattern (classified as new, switching, or add-on). The mean decrease of HbA1c was 0.9% (from 8.2% to 7.3%) in patients aged < 65 years (n = 243) versus 0.4% (from 7.4% to 7.0%) in patients aged ≥ 65 years (n = 53) (P < 0.001 and P < 0.05, respectively), with significant improvement occurring in both age groups (Fig. 4). The mean reduction of HbA1c was 0.9% (from 8.1% to 7.2%) in men (n = 166) and 0.7% (from 8.0% to 7.3%) in women (n = 135) (both P < 0.001). HbA1c decreased by 1.2% (from 8.2% to 7.0%) in patients with a baseline BMI < 25 kg/m2 (n = 61), by 0.8% (from 8.0% to 7.2%) in patients with a BMI from 25 to < 30 kg/m2 (n = 127), and by 0.6% (from 8.0% to 7.4%) in patients with a BMI ≥ 30 kg/m2 (n = 113), showing significant improvement regardless of BMI (all P<0.001). Moreover, HbA1c decreased by 0.8% (from 7.8% to 7.0%) in patients whose duration of diabetes was < 5 years (n = 81), by 1.2% (from 8.3% to 7.1%) in patients whose duration of diabetes was 5 years to < 10 years (n = 71), by 0.6% (from 8.1% to 7.5%) in patients whose duration of diabetes was 10 years to < 15 years (n = 62), and by 0.8% (from 8.2% to 7.4%) in patients whose duration of diabetes was ≥ 15 years (n = 70), showing significant improvement regardless of the duration of diabetes (all P < 0.001) [17]. HbA1c tended to increase again from week 52 onwards in patients whose duration of diabetes was ≥ 10 years. There was no significant decrease of HbA1c among newly treated patients with a relatively low baseline HbA1c (n = 33), patients with a baseline eGFR ≤ 60 mL/min/1.73 m2 (n = 35), and patients with a baseline HbA1c < 7% (n = 63), with the change of HbA1c in these subgroups being 0.4% (from 7.0% to 6.6%), 0.4% (from 7.5% to 7.1%), and 0% (from 6.5% to 6.5%), respectively (Fig. 5). However, HbA1c decreased significantly by 0.9% (from 8.2% to 7.3%, P < 0.001) in patients receiving ipragliflozin as add-on therapy (n = 241), by 1.1% (from 8.6% to 7.5%, P < 0.001) in patients switching to ipragliflozin (n = 27), by 0.9% (from 8.2% to 7.3%, P < 0.001) in patients with an eGFR ≥ 60 mL/min/1.73 m2 (n = 263), and by 0.3% (from 7.4% to 7.1%, P < 0.01) and 1.6% (from 9.4% to 7.8%, P < 0.001) in patients with a baseline HbA1c of 7% to < 8% (n = 112) and ≥ 8% (n = 126), respectively.
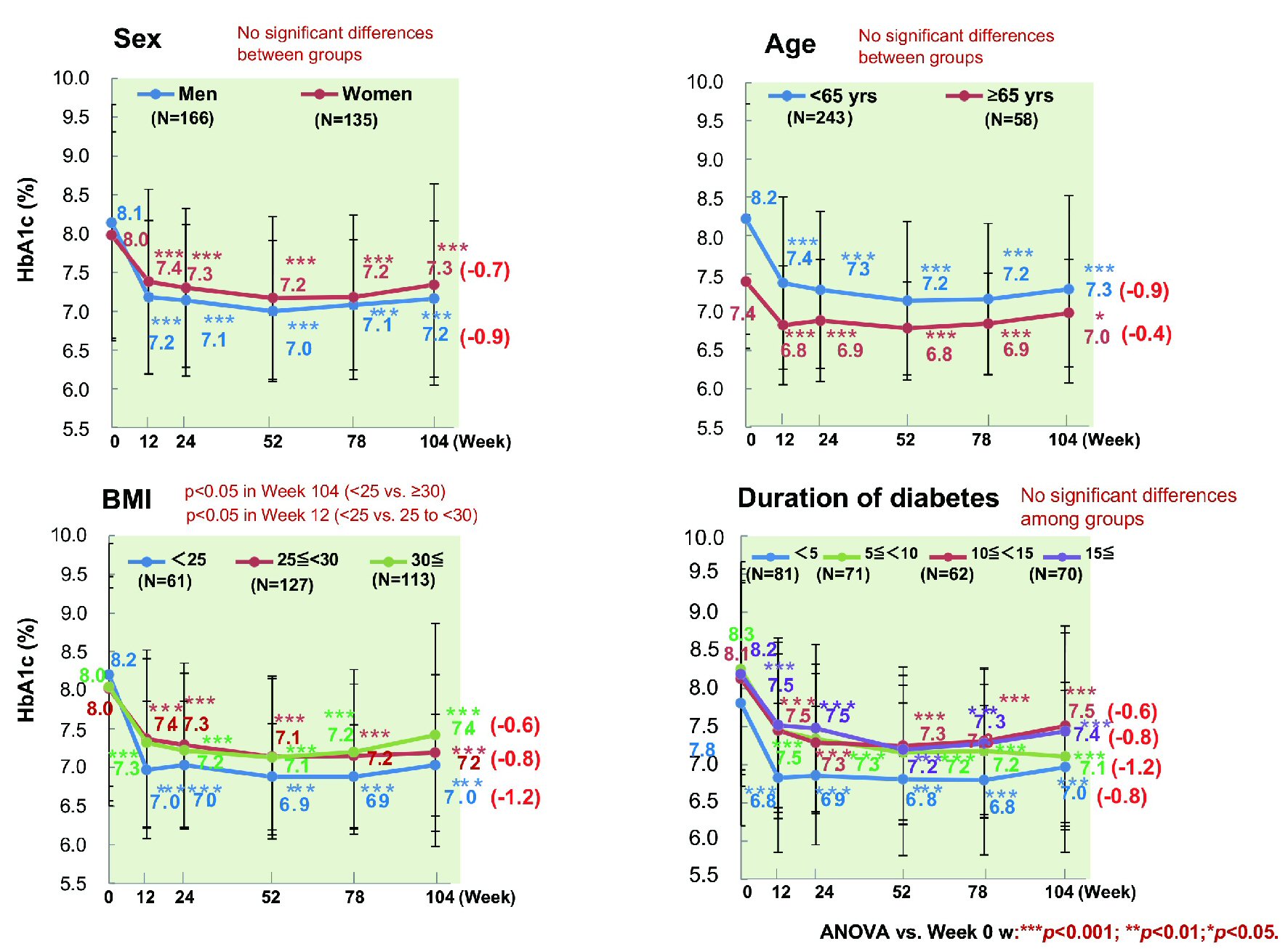 Click for large image | Figure 4. Influence of patient characteristics on HbA1c. HbA1c showed a significant decrease regardless of sex, age, disease duration, or BMI. ANOVA: analysis of variance; BMI: body mass index; HbA1c: hemoglobin A1c. |
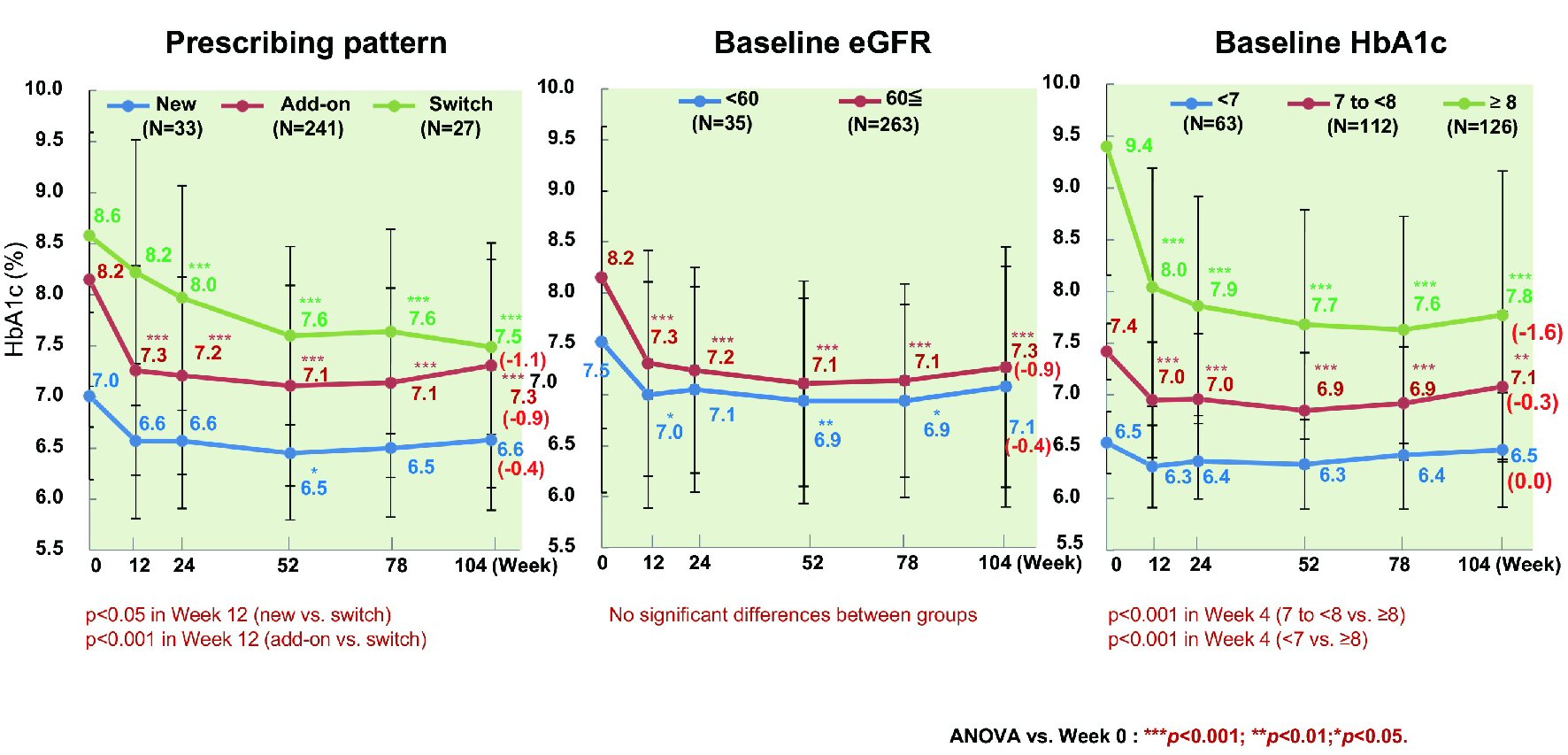 Click for large image | Figure 5. Influence of additional patient characteristics on HbA1c. In patients newly prescribed ipragliflozin, HbA1c showed a significant decrease until week 52. In patients with a baseline eGFR < 60, a significant decrease of HbA1c was observed until week 78, with no significant difference in week 104. In patients with a baseline HbA1c < 7%, no significant decrease of HbA1c was observed, and the change of HbA1c was smaller in patients with relatively low baseline values. ANOVA: analysis of variance; eGFR: estimated glomerular filtration rate; HbA1c: hemoglobin A1c. |
Analysis of HbA1c
Multiple regression analysis was conducted using the change of HbA1c from baseline to week 104 as the dependent variable and the age, sex, duration of diabetes, HbA1c, and ipragliflozin prescribing pattern as independent variables (Table 2). The standardized beta coefficient (β) for the change of HbA1c was -0.646, and a higher baseline HbA1c was associated with a larger change (P < 0.0001). Because β was 0.124 for the duration of diabetes and 0.130 for baseline BMI, the change of HbA1c was smaller as the disease duration became longer and as BMI increased (P = 0.0118 and P < 0.0088, respectively). However, sex and age did not influence the change of HbA1c in week 104 (P = 0.0874 and P = 0.5197, respectively).
 Click to view | Table 2. Multiple Regression Analysis of the Change of HbA1c in Week 104 (N = 271) |
Kidney function and microalbuminuria
From baseline to week 104, there was a significant decrease of eGFR (n = 295) by 4.5 mL/min/1.73 m2 (from 85.3 to 80.8 mL/min/1.73 m2, P < 0.001). While the overall decrease of microalbuminuria (n = 109) was not significant (from 134.1 to 136.7 mg/gCr), microalbuminuria decreased significantly by 49.5 mg/gCr (from 124.7 to 75.2 mg/gCr, P = 0.05) in patients with a baseline eGFR ≥ 60 mL/min/1.73 m2 (n = 97) (Fig. 6). In patients with baseline microalbuminuria ≤ 30 mg/gCr (n = 64), there was a nonsignificant increase of 4.3 mg/gCr (from 11.8 to 16.1 mg/gCr), while a significant decrease of 13.6 mg/gCr (from 94.0 to 80.4 mg/gCr, P < 0.001) occurred in patients with baseline microalbuminuria of 30 to < 300 mg/gCr (n = 34). In patients with baseline macroalbuminuria ≥ 300 mg/gCr (n = 11), albuminuria increased significantly by 43.0 mg/gCr (from 969.6 to 1,012.6 mg/gCr, P < 0.05).
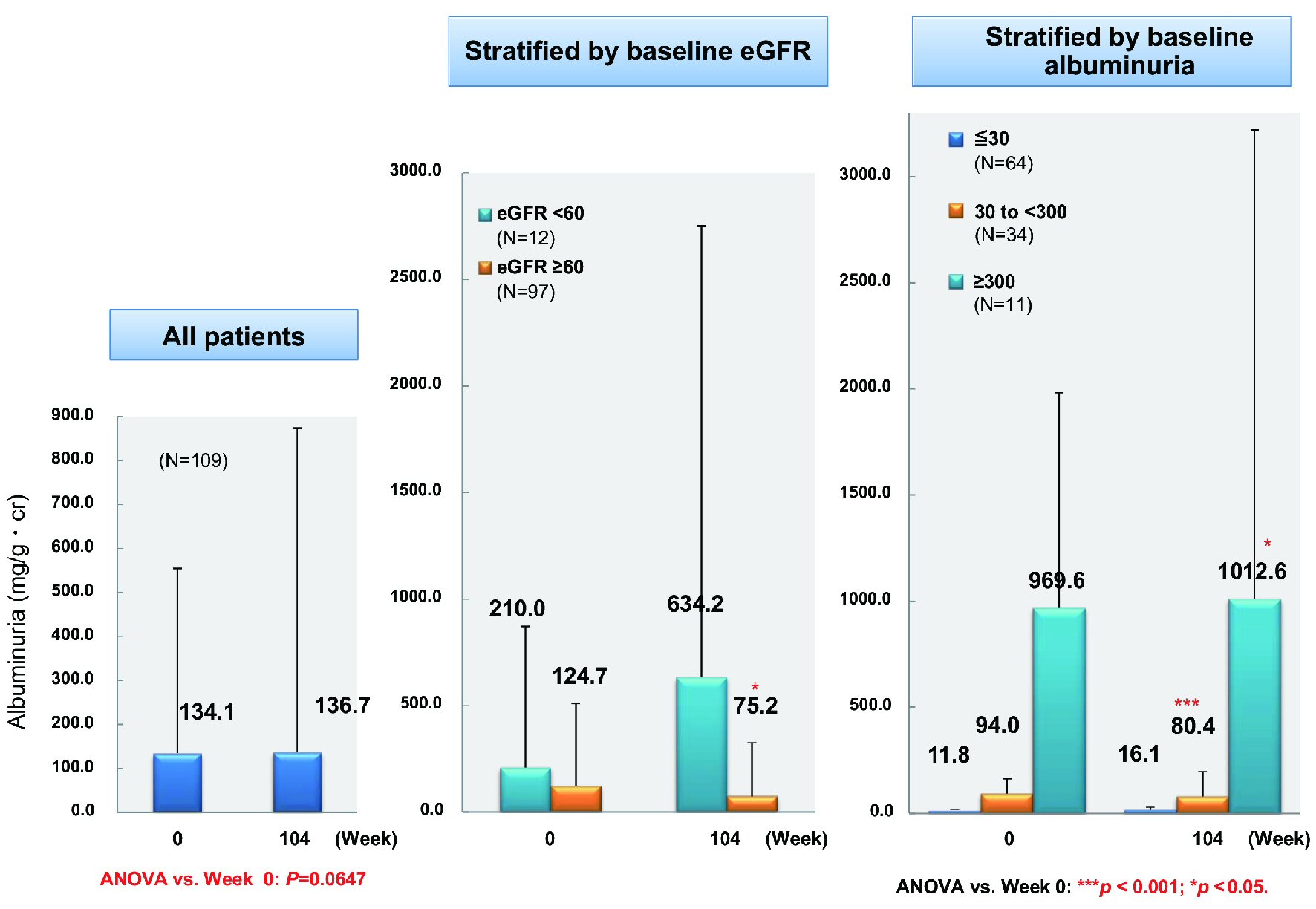 Click for large image | Figure 6. Changes of albuminuria. ANOVA: analysis of variance; eGFR: estimated glomerular filtration rate. |
Patients achieving or not achieving HbA1c < 7.0%
Excluding patients with a baseline HbA1c < 7.0% (n = 238), the proportion of patients achieving HbA1c < 7.0% peaked at 44.7% in week 52 and decreased to 34.5% in week 104. There were no significant differences of age, sex, and BMI between patients who achieved HbA1c < 7.0% (n = 132) and patients who did not (n = 169). However, there were significant differences of baseline HbA1c and the duration of diabetes (7.45% vs. 8.54%, P < 0.001 and 8.66 vs. 10.69 years, P = 0.002; data not shown). HbA1c decreased significantly by 1.03% (from 7.45% to 6.42%, P < 0.001) in patients achieving HbA1c < 7.0%, while it also decreased significantly by 0.66% (from 8.54% to 7.89%, P < 0.001) in patients not achieving HbA1c < 7.0% (Fig. 7). Patients achieving HbA1c < 7.0% did so by week 12 and their HbA1c decreased further by week 104. In contrast, HbA1c increased again without reaching < 7% in the patients not achieving this target. Patients achieving HbA1c < 7.0% also showed a significant decrease of body weight (n = 87) (by 4.1 kg from 79.5 to 75.4 kg), BMI (n = 87) (by 1.6 kg/m2 from 29.2 to 27.6 kg/m2), waist circumference (n = 116) (by 4.1 cm from 98.8 to 94.7 cm), and body fat (n = 87) (by 2.7 kg from 24.6 kg to 21.9 kg) (all P < 0.001) (Fig. 8). Likewise, patients not achieving HbA1c < 7% showed a significant decrease of body weight (n = 130) (by 2.0 kg from 78.8 to 76.8 kg), BMI (n = 130) (by 0.7 kg/m2 from 29.4 to 28.7 kg/m2), waist circumference (n = 153) (by 2.0 cm from 99.5 cm to 97.5 cm), and body fat (n = 130) (by 1.4 kg from 25.1 kg to 23.7 kg) (all P < 0.001).
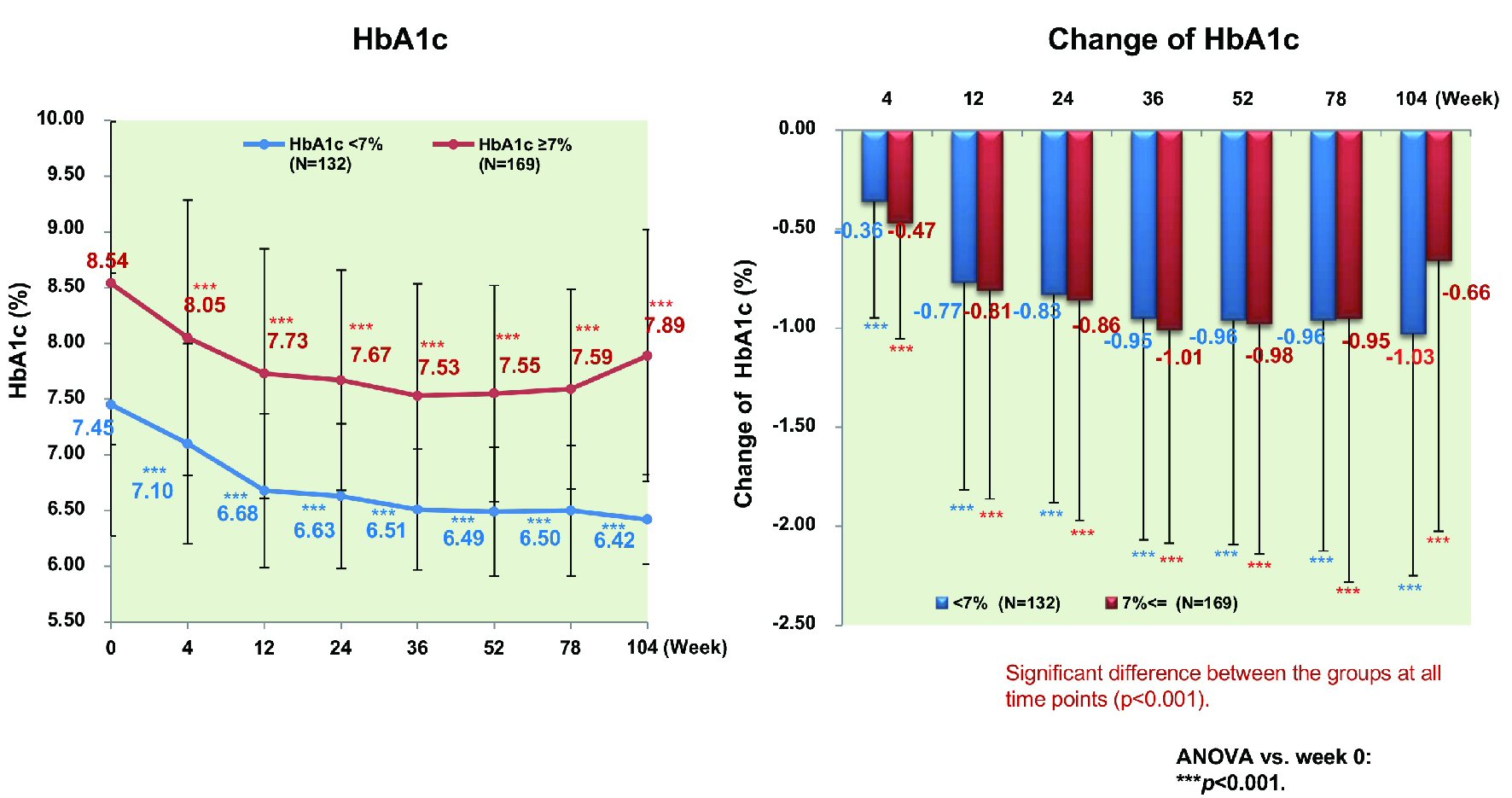 Click for large image | Figure 7. Changes of HbA1c stratified by achievement/nonachievement of HbA1c ≤ 7% in week 104. ANOVA: analysis of variance; HbA1c: hemoglobin A1c. |
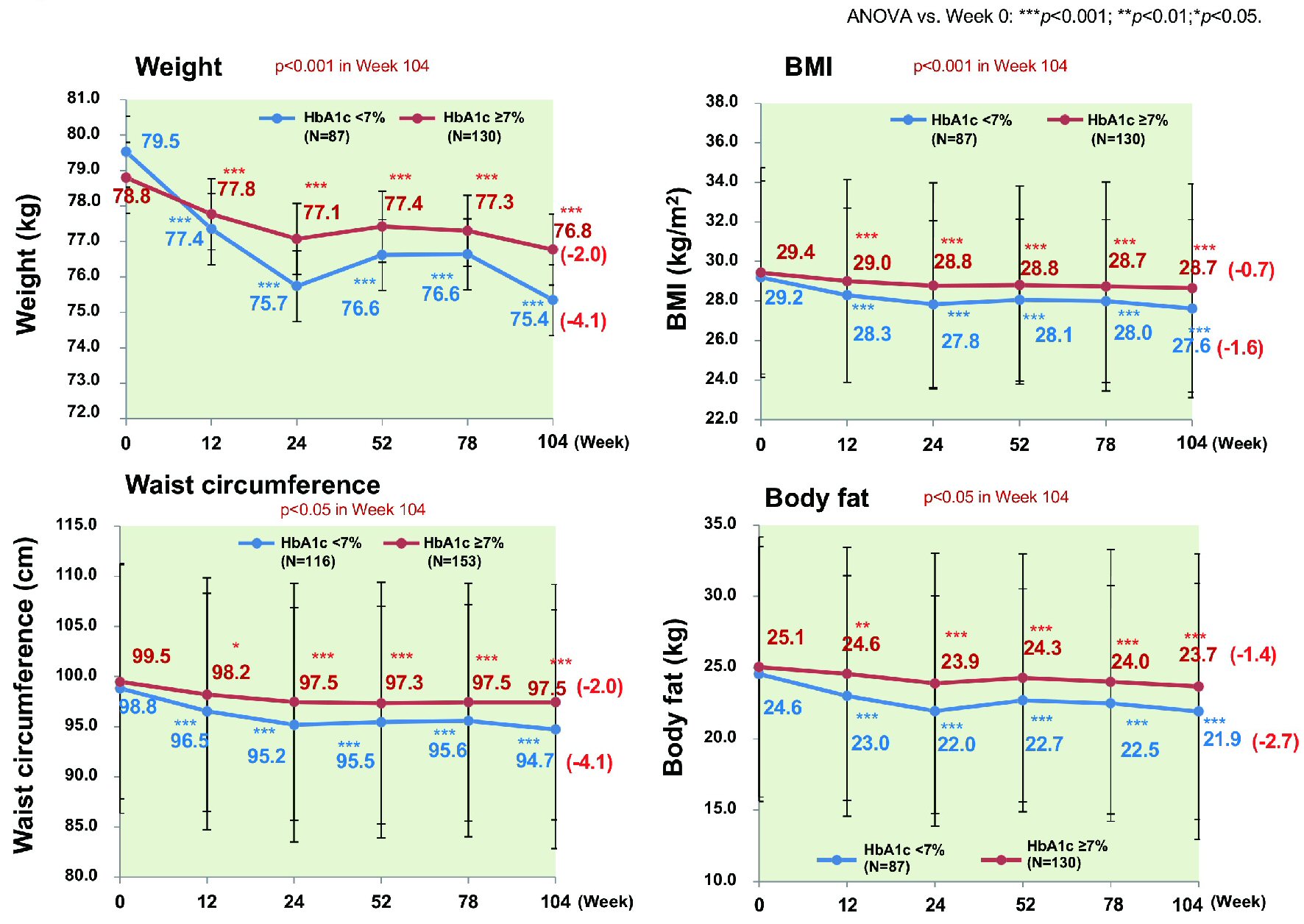 Click for large image | Figure 8. Changes of various parameters stratified by achievement/nonachievement of HbA1c ≤ 7%. Weigh, BMI, waist circumference, and body fat all decreased significantly in the patients who achieved HbA1c ≤ 7% compared with patients who did not. ANOVA: analysis of variance; BMI: body mass index. |
Aspartate aminotransferase decreased significantly in both groups (by 7.8 IU/L from 29.6 IU/L to 21.8 IU/L and by 5.0 IU/L from 34.1 IU/L to 29.0 IU/L, P < 0.001 and P < 0.01, respectively; data not shown). The decreases of these parameters in week 104 were significantly larger in patients achieving HbA1c < 7.0% than in those who did not (P < 0.001, P < 0.001, P < 0.05, P < 0.05, and P < 0.05, respectively). A higher percentage of patients achieving HbA1c < 7% complied with dietary instructions compared to those who did not.
Changes of mineral mass (data not shown)
In men (n = 108), the reduction of mineral mass was significant until week 4 (1.33%, P < 0.05) and remained around 2.0% thereafter, while significant reduction was noted until week 24 in women (n = 109). In week 104, the decrease of mineral mass was 2.16% in men and 4.26% in women (P < 0.001).
Safety
Blood ketone bodies (n = 214) increased significantly from 0.21 mmol/L to 0.31 mmol/L (P < 0.001), but suspected ketoacidosis only occurred in patients who did not suspend ipragliflozin treatment during intercurrent illness. AEs occurred in 129 of the 451 patients analyzed (28.6%), including 27 of 103 patients (26.2%) aged ≥ 65 years (Table 3). There were nine AEs in seven patients for which a causal relationship with ipragliflozin could not be excluded (Table 4). Except for one case each of “lung cancer” and “depression”, these events occurred in patients who did not suspend treatment during intercurrent illness.
 Click to view | Table 3. Summary of AEs and ARs |
 Click to view | Table 4. Serious AEs for Which a Causal Relationship With Ipragliflozin Could Not Be Excluded |
| Discussion | ▴Top |
This study investigated whether the results obtained with the SGLT2 inhibitor ipragliflozin in previous domestic clinical studies could be reproduced in the real-world clinical setting.
Fasting blood glucose was reduced significantly by approximately 20 mg/dL from baseline to week 104 of ipragliflozin treatment. On the other hand, the maximum reduction of postprandial blood glucose occurred in week 36 and postprandial glucose increased again by week 104, indicating a decline in the hypoglycemic effect of ipragliflozin over time. HbA1c showed a significant decrease in week 4 of treatment, with further significant reduction by week 12. The maximum reduction of HbA1c was noted in week 52 (0.97%), and HbA1c was slightly higher in week 104 (0.82%). This slightly weakening of the hypoglycemic effect of ipragliflozin over time was possibly related to a smaller impact on postprandial blood glucose from week 36. In patients achieving HbA1c < 7.0%, this target was reached by week 12 and HbA1c continued to decrease thereafter. Among patients not achieving HbA1c < 7.0%, the maximum reduction of HbA1c was noted in week 36 and the hypoglycemic effect of ipragliflozin decreased subsequently, with the reduction of HbA1c in week 104 being significantly smaller than in patients achieving HbA1c < 7.0%. Achieving the HbA1c target was presumably related to alleviation of glucose toxicity, improvement of insulin resistance, and recovery of pancreatic beta-cell function, while these effects of ipragliflozin may have been insufficient when HbA1c < 7% was not achieved. Interestingly, food intake increased significantly among patients not achieving the HbA1c target (data not shown), as has also been found in European research on SGLT2 inhibitors. In patients with T2D, SGLT2 expression increases in the renal tubules to enhance glucose reabsorption. We found less reduction of HbA1c as the duration of diabetes increased, suggesting that chronic upregulation of SGLT2 expression was associated with insufficient improvement of glucose toxicity, insulin resistance, and beta cell function in patients with a longer disease duration who did not achieve HbA1c < 7%. We also found that body weight, BMI, waist circumference, body fat, and aspartate aminotransferase were all significantly lower in patients achieving HbA1c < 7% compared to patients not achieving this target [18, 19]. In the former patients, improvement of insulin resistance associated with alleviation of glucose toxicity and calorie loss due to increased urinary glucose excretion presumably reduced the insulin requirement, leading to loss of visceral fat. Although we did not examine changes of concomitant drugs after initiation of ipragliflozin, it is possible that the changes of food intake and the craving for sweetness among patients not achieving HbA1c < 7% were due to hypoglycemia. However, the two groups showed no significant differences of questionnaire items suggesting hypoglycemia. A correlation between weight loss and insulin sensitivity was identified in an overseas phase II study of ipragliflozin [20]. Thus, ipragliflozin may be more effective when patients follow dietary instructions.
We detected a significant decrease of body weight from baseline to week 24 of the study. Both total body water and body fat decreased significantly until week 4, while body fat continued to decrease until week 24 with no further significant change of total body water. Therefore, weight loss up to week 4 was largely due to fluid removal by osmotic diuresis associated with increased urinary glucose excretion, while subsequent weight loss reflected fat reduction. In a previous Japanese study of ipragliflozin, approximately 50% of weight loss was due to reduction of total body fat and approximately 20% was due to loss of extracellular fluid [21]. While protein decreased significantly until week 12, there was no significant decrease subsequently and the major contributors to weight loss were reduction of total body water and body fat. In patients complying with dietary therapy, the decrease of body fat would be mainly due to visceral fat loss. Mineral mass (equivalent to bone mass) showed a significant decrease in week 104, with the percent decrease being significant to week 4 in male patients and to week 24 in female patients. Blau et al suggested that canagliflozin triggers intermittent spikes of serum phosphorus from 12 h after administration, which promote bone resorption via intermittent elevation of fibroblast growth factor 23 and parathyroid hormone levels [22]. We found a significant increase of serum phosphorus in week 104, but no significant increase of fasting phosphorous, suggesting that similar intermittent elevation of phosphorus and parathyroid hormone may be caused by ipragliflozin. However, there was no significant change of mineral mass after week 24, so parathyroid hormone may have been suppressed with reduction of urinary glucose excretion. Considering the average age of our female patients, mineral mass could have been influenced by other factors such as menopause.
As observed in other large-scale clinical studies, eGFR decreased significantly soon after initiation of ipragliflozin treatment, but there was no significant change from week 12 to week 104 [23]. SGLT2 inhibitors cause an early decline of eGFR by reducing glucose and sodium resorption in the proximal tubules to correct glomerular hypertension/hyperfiltration via normalization of tubuloglomerular feedback [24]. There was no significant improvement of microalbuminuria in patients with an eGFR < 60 mL/min/1.73 m2 or those with baseline macroalbuminuria ≥ 300 mg/gCr. Microalbuminuria may have persisted in patients with a low baseline eGFR because glomerular fibrosis had already progressed to some extent. Also, albuminuria may not have improved in patients with baseline macroalbuminuria ≥ 300 mg/gCr because their systolic blood pressure was significantly higher than in the other patients and was not decreased in week 104.
The main AEs identified in this study were urinary tract/genital infections, respiratory infections, gastrointestinal disorders, and skin disorders, which were similar to those noted in previous Japanese clinical studies of ipragliflozin and other SGLT2 inhibitors [3-9]. All serious AEs, except for lung cancer and depression, could have been avoided by suspending ipragliflozin treatment during intercurrent illness.
In conclusion, we investigated factors influencing the changes of HbA1c and body composition in 301 patients treated with ipragliflozin for 104 weeks. HbA1c showed a significantly larger decrease in patients with higher baseline HbA1c values, while the decrease was significantly smaller in patients with a longer duration of diabetes and higher baseline BMI. Weight loss was mainly due to reduction of total body water and body fat, with no significant decrease of muscle mass. Our efficacy and safety data obtained in actual clinical practice were consistent with the results of previous Japanese clinical studies, and our findings suggested that ipragliflozin is more effective in patients with a higher baseline HbA1c and shorter duration of diabetes.
Acknowledgments
ASSIGN-K is a prospective study of the Kanagawa Physicians Association that is currently in progress. We would like to thank the physicians and staff of the Kanagawa Physicians Association involved in the research and the patients participating in this study.
Financial Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper. This research was planned by the Study Group of the Diabetes Committee of Kanagawa Physicians Association and was financially supported by Astellas Pharma, Inc. The company was not involved in the study design, patient enrollment, data aggregation and analysis, data interpretation, or preparation of this report.
Conflict of Interest
None to declare.
Informed Consent
All participants provided written informed consent to this study.
Author Contributions
I.M and T.K were involved in the acquisition and analysis of data and wrote the first draft of the manuscript. I.M designed the study. I.M and T.K were involved in the statistical analysis. I.M, Y. Tanaka, and Y. Terauchi performed the model analysis. All authors reviewed and edited the manuscript critically for important intellectual content. I.M is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
| References | ▴Top |
- Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93(1):397-404.
doi pubmed - Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261(1):32-43.
doi pubmed - Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(4):382-391.
doi pubmed - Kashiwagi A, Kawano H, Kazuta K, Utsuno A, Yoshida S. Long-term safety, tolerability and efficacy of ipragliflozin in Japanese patients with type2 diabetes mellitus - IGNITE Study. Jpn Pharmacol Ther. 2015;43:85-100.
- Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30(7):1245-1255.
doi pubmed - Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, Tanizawa Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65.
doi pubmed - Inagaki N, Kondo K, Yoshinari T, Kuki H. Efficacy and safety of canagliflozin alone or as add-on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: A 52-week open-label study. J Diabetes Investig. 2015;6(2):210-218.
doi pubmed - Kaku K, Maegawa H, Tanizawa Y, Kiyosue A, Ide Y, Tokudome T, Hoshino Y, et al. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open-label study. Diabetes Ther. 2014;5(2):415-433.
doi pubmed - Kadowaki T, Haneda M, Inagaki N, Terauchi Y, Taniguchi A, Koiwai K, Rattunde H, et al. Efficacy and safety of empagliflozin monotherapy for 52 weeks in Japanese patients with type 2 diabetes: a randomized, double-blind, parallel-group study. Adv Ther. 2015;32(4):306-318.
doi pubmed - Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther. 2013;139(1):51-59.
doi pubmed - Inzucchi SE, Matthews DR, Management of Hyperglycemia in Type 2 Diabetes American Diabetes Association, European Association for the Study of Diabetes Position Statement Writing Group. Response to Comments on Inzucchi et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care 2015;38:140-149. Diabetes Care. 2015;38(8):e128-129.
doi pubmed - Kashiwagi A, Isaka H, Takinami Y, Kazuta K, Utsuno A, Yoshida S. Long-term safety, tolerability and efficacy of ipragliflozin in combination with an α-glucosidase inhibitor in Japanese patients with type 2 diabetes mellitus inadequately controlled with an α-glucosidase inhibitor alone - AGLOW Study. Jpn Pharmacol Ther. 2014;42:923-939.
- Kashiwagi A, Isaka H, Kawano H, Kazuta K, Utsuno A, Yoshida S. Long-term safety, tolerability and efficacy of ipragliflozin in combination with a dipeptidyl peptidase-4 inhibitor in Japanese patients with type 2 diabetes mellitus inadequately controlled with a dipeptidyl peptidase-4 inhibitor alone - DAYLIGHT Study. Jpn Pharmacol Ther. 21014;42:941-957.
- Kashiwagi A, Isaka H, Nakahama H, Kazuta K, Utsuno A, Yoshida S. Long-term safety, tolerability and efficacy of ipragliflozin in combination with a nateglinide in Japanese patients with type 2 diabetes mellitus inadequately controlled with nateglinide alone - CANDLE Study. Jpn Pharmacol Ther. 2014;42:959-975.
- Iizuka T, Iemitsu K, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, et al. Efficacy and safety of ipragliflozin in japanese patients with type 2 diabetes: interim outcome of the ASSIGN-K study. J Clin Med Res. 2016;8(2):116-125.
doi pubmed - Iemitsu K, Iizuka T, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, et al. Factors influencing changes in hemoglobin A1c and body weight during treatment of type 2 diabetes with ipragliflozin: interim analysis of the ASSIGN-K study. J Clin Med Res. 2016;8(5):373-378.
doi pubmed - Kashiwagi A, Yoshida S, Nakamura I, Kazuta K, Ueyama E, Takahashi H, Satomi H, et al. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes stratified by body mass index: A subgroup analysis of five randomized clinical trials. J Diabetes Investig. 2016;7(4):544-554.
doi pubmed - Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, Wasada T. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22(9):1141-1145.
doi pubmed - Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42(1):25-32.
doi pubmed - Schwartz SL, Akinlade B, Klasen S, Kowalski D, Zhang W, Wilpshaar W. Safety, pharmacokinetic, and pharmacodynamic profiles of ipragliflozin (ASP1941), a novel and selective inhibitor of sodium-dependent glucose co-transporter 2, in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13(12):1219-1227.
doi pubmed - Matsuhashi Y, Chikazawa S, Matsui J, Daimon M. Administration of SGLT-2 inhibitor ipraglifrozin improves glycemic control and reduces the body weight and fat mass. Prog Med. 2014;34:1867-1871.
doi - Blau JE, Bauman V, Conway EM, Piaggi P, Walter MF, Wright EC, Bernstein S, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3(8):e99123.
doi pubmed - Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334.
doi pubmed - Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes. 2014;38(5):356-363.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
