| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 12, Number 3, June 2022, pages 97-101
Effect of Age, Gender, and Body Mass Index on Thyroid Bethesda Classification: A Retrospective Study
Angela Achkara, b, d, Elie Naousa, b, d, e , Marie-Helene Gannage-Yareda, b, Ghassan Sleilatyb, Viviane Smayrab, c, Georges Hajjb
aEndocrinology Division, Department of Internal Medicine, Hotel Dieu de France Hospital, Beirut, Lebanon
bFaculty of Medicine, Saint-Joseph University, Beirut, Lebanon
cDepartment of Pathology, Hotel Dieu de France Hospital, Beirut, Lebanon
dThese authors contributed equally to this work.
eCorresponding Author: Elie Naous, Endocrinology Division, Department of Internal Medicine, Hotel Dieu de France Hospital, Beirut, Lebanon
Manuscript submitted April 20, 2022, accepted May 12, 2022, published online June 2, 2022
Short title: Age, Gender, BMI, and Thyroid Bethesda Score
doi: https://doi.org/10.14740/jem806
| Abstract | ▴Top |
Background: Thyroid cancer incidence is increasing nowadays. Bethesda system classifies fine needle aspiration (FNA) cytology into six categories linked to cancer risk. Several papers have studied relationships between thyroid pathology results and age, gender, and body mass index (BMI), but very few have taken into consideration the Bethesda score. The aim of this study was to assess the relationship between the Bethesda system and age, gender, and BMI.
Methods: All the patients who underwent an ultrasound-guided FNA of a thyroid nodule between November 2017 and September 2020 were included in this study (668 subjects, 80.7% women, mean age 50.1 ± 13.3 years). Bethesda classification results, age, gender, and BMI were collected from the archives of the institution’s Pathology Department.
Results: The observed proportions of the incremental Bethesda classes (1 through 6) were respectively 13.3%, 57.0%, 15.7%, 6.1%, 4.9%, and 2.8%. Bethesda score was negatively correlated with age (Spearman’s rho = -0.106 (95% confidence interval (CI): -0.180; -0.026), P value = 0.006). The ratio men/women increased with increasing Bethesda score (linear trend P value = 0.043). Bethesda score was not correlated with BMI (Spearman’s rho = 0.049 (95% CI: -0.033; 0.120), P value = 0.21), even after adjustment for gender and age.
Conclusions: Bethesda scores decreases with age and are not associated with gender; however, men/women ratio increases with increasing scores. BMI does not correlate with higher Bethesda scores. More studies should be performed to identify factors that might influence Bethesda score.
Keywords: Bethesda score; Thyroid cancer; Body mass index; Gender; Age
| Introduction | ▴Top |
Thyroid cancer is the most prevalent cancer in endocrinology [1]. In the USA, it ranked 12th in terms of incidence, with an estimate of 52,890 new cases in 2020 [2]. It is one of the cancers to have had the most rapid increase of its prevalence in the last years. This could partially be explained by the improvement of the screening methods [3]. However, many environmental and biological factors can contribute to this phenomenon: exposure to ionizing radiation, diet low in iodine, estrogen treatment, Hashimoto’s thyroiditis and heredity [4]. Obesity has also been proposed to be an additional risk factor, but its association with thyroid cancer remains controversial [5-11]. Thyroid cancer is three to four times more common in women than in men [12]. Even if it can occur at any age, about two-thirds of all cases are found between the ages of 20 and 55 [13].
The thyroid Bethesda system classifies fine needle aspiration (FNA) cytology of a thyroid nodule into six categories, each category being linked to a certain malignancy risk [14]. Several papers have studied the relationships between thyroid pathology results and age [12, 15], gender [8], and body mass index (BMI) [6, 10], but very few have taken into consideration the Bethesda classification [11, 16]. An inverse relationship was found between BMI and Bethesda classification in one study [11]. Another study has shown that although thyroid nodules increase with age, Bethesda FNA cytology results showed lower scores with increased age [16]. To the best of our knowledge, no studies have yet evaluated the relationship between Bethesda classification and gender. The purpose of this study was to clarify the relationship between Bethesda classification and age, gender, and BMI.
| Materials and Methods | ▴Top |
In this retrospective study, all the subjects with no exclusions, who underwent an ultrasound-guided FNA of a thyroid nodule between November 2017 and September 2020 at our institution were included. The FNA material was air-dried, stained with May-Grunwald Giemsa stain, and interpreted by experienced cytologists.
The collected data were the following: gender, age (in years), weight (in kilograms), height (in meters) and BMI (expressed in kg/m2) as well as Bethesda classification results. BMI was calculated as the weight in kilograms divided by the square of height in meters.
Patients were stratified in subgroups according to their BMI: underweight (BMI < 18 kg/m2), normal weight (18 < BMI < 25 kg/m2), overweight (25 < BMI < 30 kg/m2), and obese (> 30 kg/m2).
The Bethesda classification was reported as a score from 1 to 6, using the 2017 classification: 1 for non-diagnostic or unsatisfactory, 2 for benign, 3 for atypia of undetermined significance or follicular lesion of undetermined significance, 4 for follicular neoplasm or suspicious for a follicular neoplasm, 5 for suspicious of malignancy, and 6 for malignant. This study was approved by the Ethics Committee of Saint Joseph University in Beirut (Tfem/2020/06) and was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Statistics
The distribution of BMI and age was studied using the Shapiro-Wilk test of normality and quantile-quantile plots. Categorical and ordinal data were presented as frequencies, percentage and their 95% confidence interval (CI). The correlation between BMI, age, and Bethesda classification was performed using the non-parametric Spearman correlation coefficient, with its 95% CI calculated by the bootstrapping method performed on 10,000 random samples. For the comparison between the two sexes, Student’s t-test was used for age, and the Mann-Whitney U test was used for height, weight, and BMI. The comparison of BMI categories and the Bethesda classification subgroups was performed using the Pearson Chi2 test. All the calculations were performed using SPSS v26.1 software (IBM Corp., released 2019, SPSS Statistics for Windows Version 26.1, Armonk, NY).
| Results | ▴Top |
Patient’s characteristics
During the specified period, 668 subjects (80.7% women) were included in this study. Their mean age was 50.1 ± 13.3 years and their median BMI was 24.8 (interquartile range (IQR): 22.0 - 27.7) kg/m2. Mean age was not different between men (51.3 ± 13.7) and women (49.8 ± 13.2), P value = 0.26. Men had a higher median BMI compared to women (27.5 (IQR: 25.3 - 30.0) vs. 24.2 (IQR 21.5 - 26.9), P value < 0.001, respectively).
Relationship between Bethesda classification and age, gender, and BMI
The observed proportions of the incremental Bethesda classes (1 through 6) in the sample were 13.4%, 57.0%, 15.7%, 6.1%, 4.9%, and 2.8%, respectively. Bethesda classification is negatively correlated with age in the overall sample (Spearman’s rho = -0.106 (95% CI: -0.180; -0.026), P value = 0.006), as depicted in Figure 1, and in the women subgroup (Spearman’ rho = -0.119 (95% CI: -0.212; -0.023), P value = 0.006).
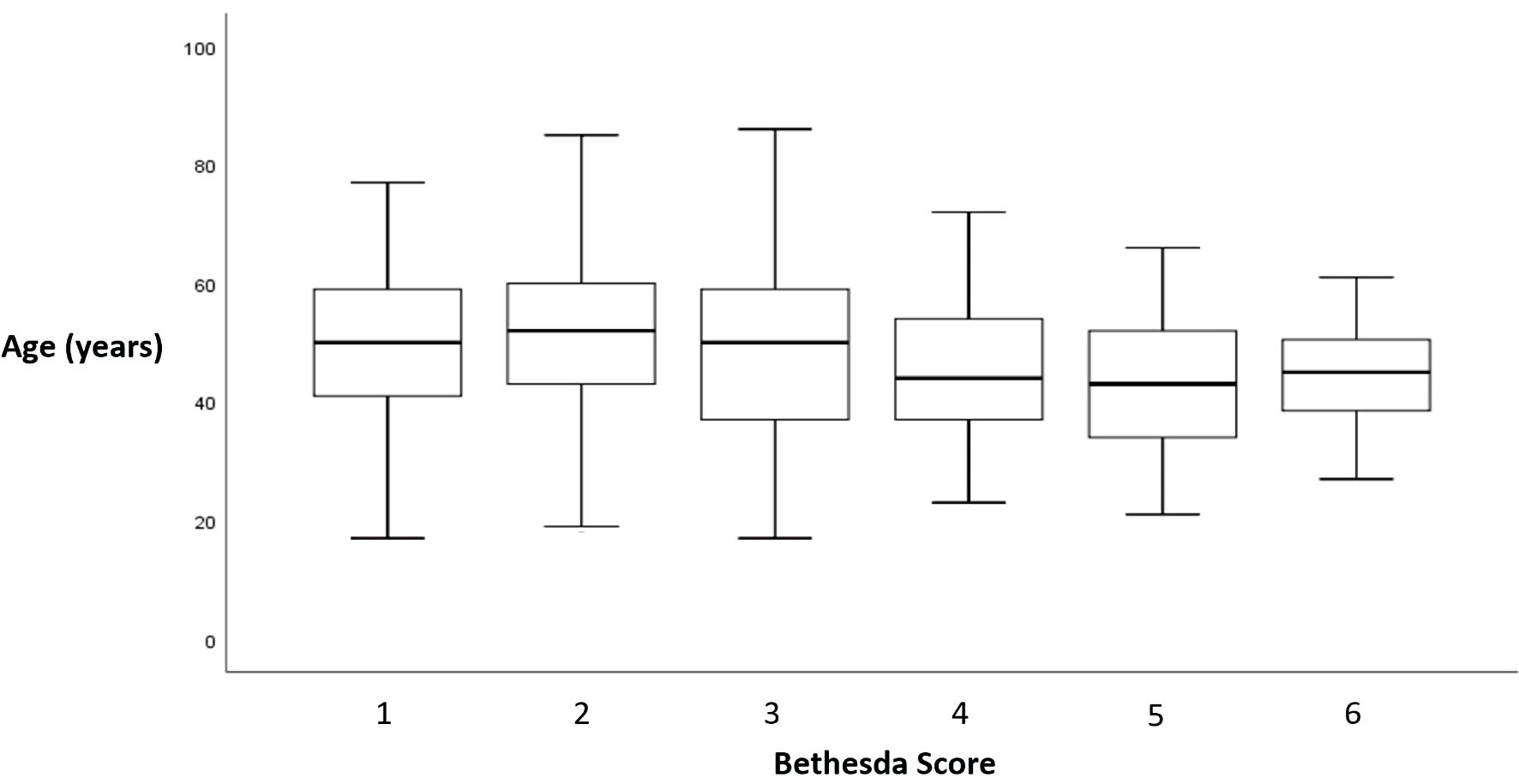 Click for large image | Figure 1. Relationship between Bethesda score and age. Bethesda classification was reported as a score from 1 to 6: 1 for non-diagnostic or unsatisfactory, 2 for benign, 3 for atypia of undetermined significance or follicular lesion of undetermined significance, 4 for follicular neoplasm or suspicious for a follicular neoplasm, 5 for suspicious of malignancy, and 6 for malignant. |
The median Bethesda class was similar in men compared to women (respectively 2 (2 - 3) vs. 2 (2 - 3), P value = 0.517). Table 1 shows the proportion of women and men in each Bethesda category. The ratio men/women tends to increase with Bethesda classes (Mantel-Haenszel linear trend test, P value = 0.043).
 Click to view | Table 1. Distribution of Bethesda Score According to Gender |
The relation between Bethesda classes and BMI is shown in Figure 2. Table 2 shows the distribution of Bethesda class and BMI categories. There is neither correlation between Bethesda classification and BMI categories separately (P value = 0.27), nor with BMI (Spearman’s rho = 0.049 (95% CI: -0.033; 0.120), P value = 0.21). The correlation between Bethesda classes and BMI is also nonsignificant when the analysis was performed separately in men and women (P value = 0.20 and P value = 0.51, respectively).
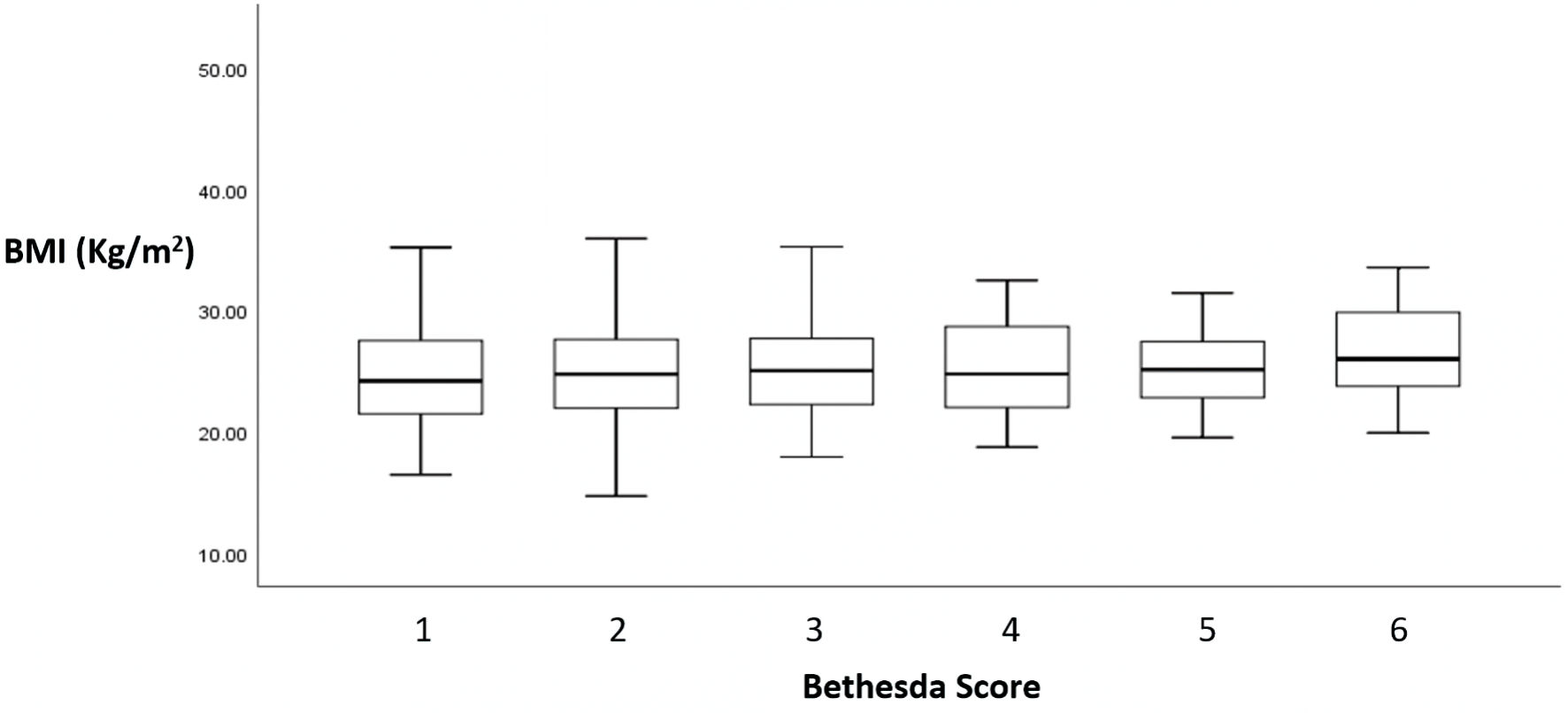 Click for large image | Figure 2. Relationship between Bethesda score and BMI. Bethesda classification was reported as a score from 1 to 6: 1 for non-diagnostic or unsatisfactory, 2 for benign, 3 for atypia of undetermined significance or follicular lesion of undetermined significance, 4 for follicular neoplasm or suspicious for a follicular neoplasm, 5 for suspicious of malignancy, and 6 malignant. |
 Click to view | Table 2. Distribution of Bethesda Score According to BMI Categories |
| Discussion | ▴Top |
While the current series did not show a correlation between Bethesda classification and BMI, Bethesda classes were inversely correlated with age in the overall population and particularly in women. In addition, the men/women ratio tends to increase with Bethesda classes.
From an epidemiological standpoint, prevalence of thyroid nodules increases with age [17], from a mean of 1.5 nodules in young individuals (20 - 30 years) to 2.2 nodules in elder individuals aged more than 70. However, the risk of malignancy of a newly identified nodule declines with age [16], a finding reproduced in the current series, while a U-shaped pattern [12], and no age trends were described by others [15]. Additionally, the gender difference we observed suggests that women, contrary to men, are less prone to a malignant cytology with age.
Median Bethesda class was similar between men and women in the current series; however, the proportion of men increased with Bethesda classes. Other series found that despite the higher prevalence of thyroid nodules in women the risk of malignancy on a nodule’s FNA cytology is higher in men [12, 18]. This finding was also supported by Kwon et al [8] who attributed this increase in thyroid cancer risk to metabolic obesity in men. Moreover, it has been shown that testosterone regulates thyroid cancer progression by reducing tumor suppressor gene expression and tumor immunity [19].
Since the results of the current series did not find any correlation between BMI and Bethesda classification, even after adjustments for age and sex, the role of obesity as a risk factor for thyroid cancer is questioned. This finding refutes the ecological fallacy that presumably correlates the increased prevalence of thyroid cancer with the worldwide increase of obesity prevalence in all age groups [20]. Instead, the apparent increase in thyroid cancer prevalence is explained, at least partially, by better screening [21], while not excluding environmental and biological factors [22]. The debate is still open: in one of two recent meta-analyses performed on 21 observational studies [6, 23], Schmid et al found respectively a 25% and 55% greater risk of thyroid cancer in overweight and obese individuals compared with their normal-weight peers [6]. However, Fussey et al, using Mendelian randomization scheme, did not plead for a causal role for obesity neither in benign nodular thyroid disease nor in thyroid cancer [10].
The relationship between obesity and FNA cytology was reported in two studies. In the first one, a retrospective study, obesity has not been shown to modify the risk of differentiated thyroid cancer [11]. Surprisingly, in this study, the authors found a significantly lower rate of malignancy suspicion or malignant cytology in women with severe obesity (BMI > 35 kg/m2). At the opposite, in the second study, Arduc et al [24] found that patients with high BMI (> 30 kg/m2) had a 3.8-fold higher risk of malignant thyroid nodule on FNA compared to patients with lower BMI.
Strengths and limitations
The current sample, through the men/women (1/4) sex ratio, is representative of the underlying population encountered in the literature [15, 25]. In addition, the preponderance of Bethesda categories 0, 1, and 2, which constituted 85% of cytology results, is in accordance with previously reported studies [15, 26]. The relatively smaller sample size of the men subgroup may have underpowered the statistical comparisons in the subgroup and led to nonsignificant results. Finally, being cross-sectional by design constitutes a limitation to firmly establish the association of BMI, age, and gender with Bethesda scores. Thus, more scientifically rigorous studies are needed to confirm these associations.
Conclusions
The current series showed no evidence for a correlation between obesity and the cytological results of FNA, even after adjusting for sex and age, pointing towards the absence of correlation between thyroid cancer and BMI. Bethesda classes were inversely correlated with age and did not significantly differ with gender even though the proportion of men increased with higher classes. More prospective studies are needed to better understand the role of potential factors that might explain Bethesda class variation.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received to assist with the preparation of this manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
This was a retrospective study; patient’s data were retrieved from the archive of the pathology laboratory. Thus, no informed consent was needed.
Author Contributions
All authors contributed to the writing and revision of the manuscript. AA and EN contributed equally to conducting the study and writing the manuscript. AA, EN, MHGY, GS, VS and GH were responsible for the final version of the manuscript. All authors read and approved the final manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Lebastchi AH, Callender GG. Thyroid cancer. Curr Probl Cancer. 2014;38(2):48-74.
doi pubmed - Cancer of the thyroid - Cancer Stat Facts [Internet]. SEER. cited Apr 23, 2020. Available from: https://seer.cancer.gov/statfacts/html/thyro.html.
- Force USPST, Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, et al. Screening for thyroid cancer: US preventive services task force recommendation statement. JAMA. 2017;317(18):1882-1887.
doi pubmed - Liu Y, Su L, Xiao H. Review of factors related to the thyroid cancer epidemic. Int J Endocrinol. 2017;2017:5308635.
doi pubmed - Handelsman RS, Alvarez AL, Picado O, Farra JC, Lew JI. Inverse relationship of BMI to TSH and risk of papillary thyroid cancer in surgical patients. J Surg Res. 2019;244:96-101.
doi pubmed - Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev. 2015;16(12):1042-1054.
doi pubmed - Pazaitou-Panayiotou K, Polyzos SA, Mantzoros CS. Obesity and thyroid cancer: epidemiologic associations and underlying mechanisms. Obes Rev. 2013;14(12):1006-1022.
doi pubmed - Kwon H, Chang Y, Cho A, Ahn J, Park SE, Park CY, Lee WY, et al. Metabolic obesity phenotypes and thyroid cancer risk: a cohort study. Thyroid. 2019;29(3):349-358.
doi pubmed - Bradley D. Obesity, Thyroid nodularity, and thyroid cancer: epiphenomenon or cause? J Clin Endocrinol Metab. 2020;105(8):e3010-e3012.
doi pubmed - Fussey JM, Beaumont RN, Wood AR, Vaidya B, Smith J, Tyrrell J. Does obesity cause thyroid cancer? A mendelian randomization study. J Clin Endocrinol Metab. 2020;105(7):e2398-2407.
doi pubmed - Rotondi M, Castagna MG, Cappelli C, Ciuoli C, Coperchini F, Chiofalo F, Maino F, et al. Obesity does not modify the risk of differentiated thyroid cancer in a cytological series of thyroid nodules. Eur Thyroid J. 2016;5(2):125-131.
doi pubmed - Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med. 1992;93(4):363-369.
doi - Thyroid cancer - Risk Factors [Internet]. Cancer.Net. 2012. Available from: https://www.cancer.net/cancer-types/thyroid-cancer/risk-factors.
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341-1346.
doi pubmed - Joseph-Auguste J, Lin L, Demar M, Duffas O, Molinie V, Sulpicy C, Dorival MJ, et al. Epidemiologic, clinical, ultrasonographic, and cytological features of thyroid nodules in predicting malignancy risk: a retrospective study of 442 French Afro-Caribbean patients. Int J Endocrinol. 2020;2020:4039290.
doi pubmed - Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, Krane JF, et al. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab. 2015;100(12):4434-4440.
doi pubmed - Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22(6):901-911.
doi pubmed - Godoi Cavalheiro B, Kober Nogueira Leite A, Luongo de Matos L, Palermo Miazaki A, Marcel Ientile J, Aurelio VKM, Roberto Cernea C. Malignancy rates in thyroid nodules classified as Bethesda categories III and IV: retrospective data from a tertiary center. Int J Endocrinol Metab. 2018;16(1):e12871.
doi pubmed - Zhang LJ, Xiong Y, Nilubol N, He M, Bommareddi S, Zhu X, Jia L, et al. Testosterone regulates thyroid cancer progression by modifying tumor suppressor genes and tumor immunity. Carcinogenesis. 2015;36(4):420-428.
doi pubmed - Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1-17.
doi pubmed - Kim TY, Kim WG, Kim WB, Shong YK. Current status and future perspectives in differentiated thyroid cancer. Endocrinol Metab (Seoul). 2014;29(3):217-225.
doi pubmed - Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115(16):3801-3807.
doi pubmed - Ma J, Huang M, Wang L, Ye W, Tong Y, Wang H. Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Med Sci Monit. 2015;21:283-291.
doi pubmed - Arduc A, Dogan BA, Tuna MM, Tutuncu Y, Isik S, Berker D, Guler S. Higher body mass index and larger waist circumference may be predictors of thyroid carcinoma in patients with Hurthle-cell lesion/neoplasm fine-needle aspiration diagnosis. Clin Endocrinol (Oxf). 2015;83(3):405-411.
doi pubmed - Yaprak Bayrak B, Eruyar AT. Malignancy rates for Bethesda III and IV thyroid nodules: a retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC Endocr Disord. 2020;20(1):48.
doi pubmed - Thewjitcharoen Y, Butadej S, Nakasatien S, Chotwanvirat P, Porramatikul S, Krittiyawong S, Lekpittaya N, et al. Incidence and malignancy rates classified by the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) - An 8-year tertiary center experience in Thailand. J Clin Transl Endocrinol. 2019;16:100175.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
