| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 12, Number 3, June 2022, pages 111-115
Hypocalcemia After the Administration of Denosumab in a Patient With Osteoporotic Fracture and Vitamin D Deficiency
Dohee Kim
Division of Endocrinology, Department of Internal Medicine, Dankook University College of Medicine, Dongnam-gu, Cheonan-si, Chungnam 330-714, Korea
Manuscript submitted May 2, 2022, accepted June 2, 2022, published online June 16, 2022
Short title: Denosumab-Associated Hypocalcemia
doi: https://doi.org/10.14740/jem810
| Abstract | ▴Top |
Denosumab is a potent antiresorptive agent used for the treatment of osteoporosis and the prevention of skeletal-related events in patients with multiple myeloma or bone metastasis from solid tumors. Although denosumab generally seems to be safe and well tolerated, denosumab-associated hypocalcemia may occur, and the majority of cases are asymptomatic or mild. Underlying malignancy, renal failure, calcium or vitamin D deficiency, and high bone turnover have been reported as risk factors for hypocalcemia. Here, the author reports a case of hypocalcemia and hypophosphatemia following the administration of denosumab for the treatment of osteoporotic fracture in a patient with underlying but unrecognized severe vitamin D deficiency. Following the supplementation of calcium, vitamin D, and phosphorus, serum calcium and phosphorus levels returned to normal levels after about 2 weeks. To avoid denosumab-associated hypocalcemia, calcium and vitamin D status, as well as renal function should be assessed and corrected if appropriate.
Keywords: Denosumab; Hypocalcemia; Osteoporosis; Hypophosphatemia
| Introduction | ▴Top |
Denosumab is a fully human monoclonal antibody that binds to the receptor activator of nuclear factor kappa-B (RANK) ligand, thereby reducing osteoclast number and activity and resulting in decreased bone resorption [1]. In a long-term clinical study, denosumab at a dose of 60 mg every 6 months reduced bone turnover markers, increased bone mineral density (BMD), and reduced the risk of new vertebral and nonvertebral fractures including hip fractures for up to 10 years, with a favorable safety profile [1]. Denosumab, as well as bisphosphates, has been recommended as an initial treatment in postmenopausal women with osteoporosis at high risk of fractures [2, 3]. Denosumab was also approved to be used for the prevention of skeletal-related events (SREs) in patients with multiple myeloma or bone metastases from solid tumors, being superior to zoledronic acid [4, 5].
Despite this demonstrated efficacy, several rare but serious adverse events related to denosumab therapy include hypocalcemia, osteonecrosis of the jaw, and atypical femur fractures [4]. The incidence of denosumab-associated hypocalcemia has been reported as 2-20% of women with postmenopausal osteoporosis and 10-50% of bone metastasis patients who were administered a single 120 mg dose every 4 weeks [6]. Hypocalcemia is more frequent with denosumab than with zoledronic acid (9.6% vs. 5.2%) [4]. Most hypocalcemic events are asymptomatic or mild, especially in patients with osteoporosis [4, 7]. Hypocalcemia usually occurs 1 - 2 weeks after the administration of denosumab, to correspond to the nadir of its action [7]. In general, the risk for denosumab-associated hypocalcemia is greater in osteoporosis patients with renal impairment, vitamin D deficiency, and a lack of calcium and/or vitamin D supplementation [7, 8]. Prior to denosumab administration, it is recommended that pretreatment serum calcium and 25-hydroxyvitamin D (25(OH)D) levels are measured and deficiencies are corrected to prevent hypocalcemia [4].
Here, the author reports a case of hypocalcemia following the administration of denosumab for the treatment of osteoporotic fracture in a patient with severe vitamin D deficiency.
| Case Report | ▴Top |
Investigations
An 88-year-old woman was referred for workup and treatment of hypocalcemia from the department of rehabilitation medicine. She was admitted and operated on for left hip intertrochanteric fracture after a slip down injury 12 days previously and had been administered with denosumab 60 mg subcutaneous injection for osteoporotic fracture 5 days previously in the department of orthopedic surgery. Thereafter, she was transferred for physical therapy and rehabilitation to regain normal range of motion. She was incidentally found to be hypocalcemic to 5.7 mg/dL (8.6 - 10.2) in routine chemistry which was not checked prior to denosumab administration. Additionally, compression fractures in T12 - L3 vertebral bodies and status post vertebroplasty in T12 and L1 vertebrae were observed in chest and abdominal computed tomography (CT). BMD (dual energy X-ray absorptiometry, Hologic) showed the T-score was -4.0 in the lumbar spine (L2 - 4), -3.7 in the femoral neck, and -2.8 in total hip. She had hypertension, Parkinson’s disease, and dementia. She had been taking isoniazid, rifampin, and ethambutol after surgery for tuberculosis-induced abscess in the left parotid gland for 4 months and her serum calcium levels were normal 4 months previously. Before this admission, she could ambulate independently but had been staying inside her house because of her medical condition and the coronavirus disease 2019 (COVID-19) pandemic. Although she could communicate simply previously, she did not talk or communicate after the hip operation and had no focal lesions in brain magnetic resonance imaging (MRI). She did not present any clinical manifestations including Chvostek’s sign or Trousseau’s sign.
Diagnosis
She had serum total calcium 5.5 mg/dL, albumin 2.9 g/dL (3.5 - 5.2), corrected calcium 6.4 mg/dL, phosphorus 1.0 mg/dL (2.5 - 4.5), alkaline phosphatase 115 U/L (35 - 105), creatinine 0.68 mg/dL (0.51 - 0.95), intact parathyroid hormone (PTH) 150.5 pg/mL (8 - 76), 25(OH)D 3.5 ng/mL, 1,25-dihydroxyvitamin D (1,25(OH)2D) 7.16 pg/mL (19.6 - 54.3), and magnesium 2.13 mg/dL (1.6 - 2.4). The 24-h urine laboratory results were the following: calcium 45.54 mg/day (100 - 300), phosphorus 0.52 g/day (0.4 - 1.3), creatinine 0.55 g/day (0.74 - 1.57), and tubular reabsorption of phosphorus (TRP) 62%. Laboratory findings indicated that hypocalcemia and hypophosphatemia were caused and aggravated by severe vitamin D deficiency and the administration of denosumab.
Treatment
She was soon treated with intravenous (IV) calcium and phosphorus and then oral calcium and calcitriol replacement. Her corrected serum calcium levels were 8.8 mg/dL, phosphorus 1.7 mg/dL with calcitriol 0.5 µg/day, oral elemental calcium 1.8 g/day, and IV phosphorus replacement 2 weeks after detection of hypocalcemia (Fig. 1). Thereafter, she was discharged on calcitriol 1 µg/day and oral calcium 1.2 g/day without IV phosphorus replacement and her corrected serum calcium levels were improved to 8.9 mg/dL and phosphorus to 2.5 mg/dL.
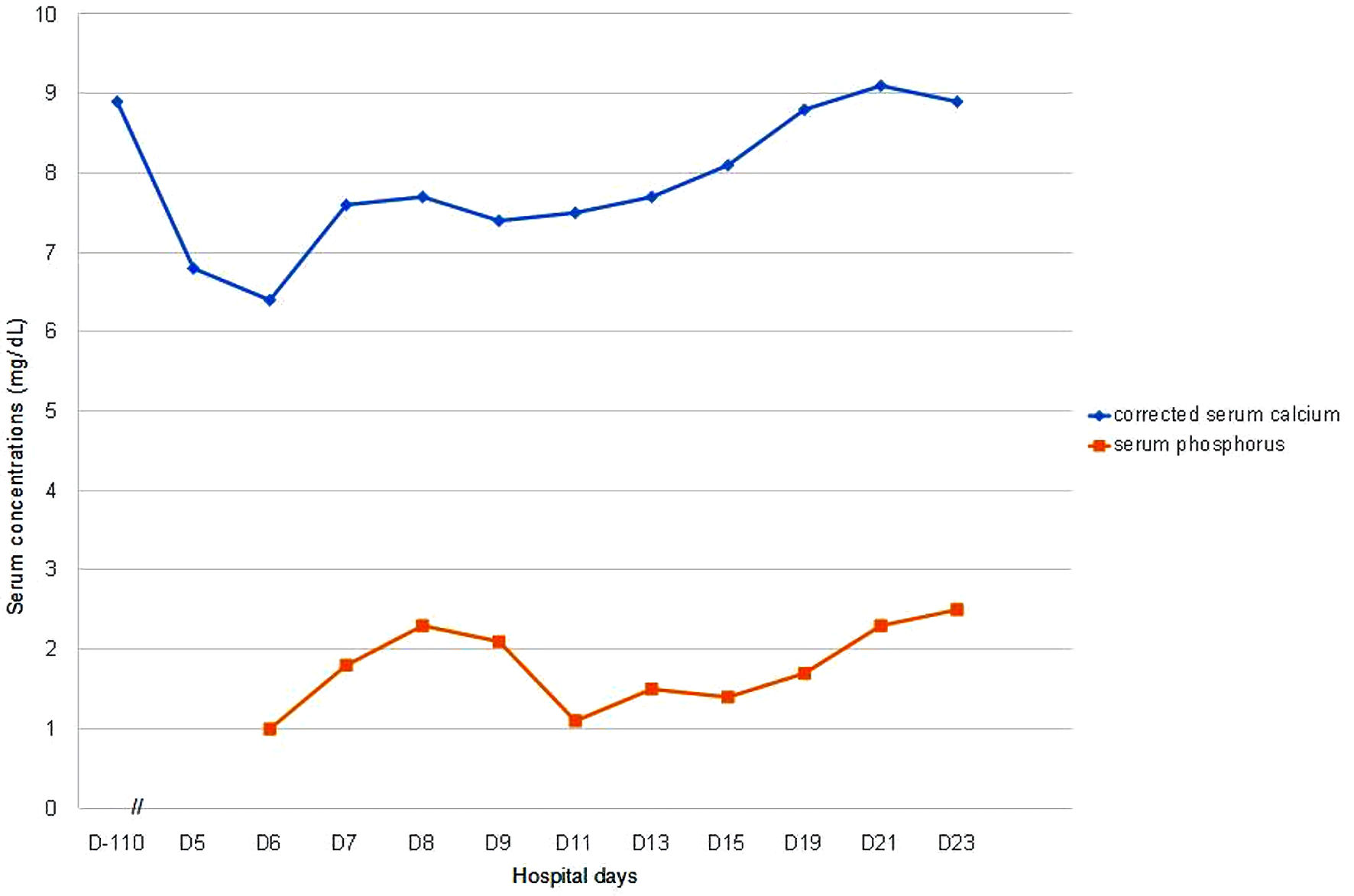 Click for large image | Figure 1. Serum corrected calcium and phosphorus levels in a patient with hypocalcemia after denosumab therapy. D-110 means 110 days before denosumab was administered and serum calcium was normal. D0 means the day when denosumab was administered, and serum calcium was not checked on D0, so there is no “D0” in the figure. D5 is the first day when low serum calcium was noticed after denosumab administration. |
Follow-up and outcomes
One month after discharge, she was taking only cholecalciferol 1,000 IU/day and oral calcium 500 mg/day. Thereafter, she was transferred to another long-term care hospital near her home.
| Discussion | ▴Top |
Denosumab is a preferred therapeutic agent for osteoporosis and metastatic bone disease due to its superior efficacy and better tolerability with the advantages of no known renal toxicity, convenient administration via subcutaneous route, and fewer acute-phase reactions compared with bisphosphonates including the potent zoledronic acid [4, 9]. Although long-term therapy with denosumab is generally well tolerated, patients are at increased risk of rare but important adverse events including hypocalcemia, most cases of which are asymptomatic and mild [4].
In a systematic review of seven trials including a total of 8,990 cancer patients receiving denosumab, the overall incidences of all-grade (serum calcium < 8.0 mg/dL) and high-grade (serum calcium < 7.0 mg/dL) hypocalcemia were 5.2% and 2.0%, respectively [10]. Compared to controls receiving either placebo or bisphosphonate, the use of denosumab was associated with a significantly increased risk of developing all-grade (relative risk (RR) of 1.93) and high-grade hypocalcemia (RR 4.03) [10]. Three pivotal phase III trials in 5,677 cancer patients with bone metastases showed that hypocalcemia was more commonly reported with denosumab than with zoledronic acid (9.6% vs. 5.0%) [11]. A retrospective cohort study of cancer patients with bone metastasis demonstrated that the incidence of hypocalcemia with denosumab was higher than that in the zoledronic acid group (5.5% vs. 3.1%) [8]. In another retrospective analysis of patient-level data from three phase III trials including 5,723 patients with metastatic bone disease, the overall incidence of laboratory events of hypocalcemia (serum calcium < 8.0 mg/dL) was higher with denosumab (12.4%) than with zoledronic acid (5.3%) [12]. Hypocalcemia occurred earlier for denosumab-treated than for zoledronic acid-treated patients (median 3.8 months vs. 6.5 months). Calcium and/or vitamin D supplementation led to a 40% reduction of denosumab-associated hypocalcemia [12].
Although randomized controlled trials have reported a 0.05-1.7% rate of hypocalcemia in denosumab-treated postmenopausal women with osteoporosis, retrospective analysis from 2,005 osteoporotic community-dwelling patients treated with denosumab showed a 7.4% rate of hypocalcemia (1% less than 8 mg/dL) [13]. In a real-world cohort study of 790 postmenopausal osteoporotic patients who received denosumab, serum calcium < 8.5 mg/dL and < 8.0 mg/dL were observed in 8.2% and 1.0% of patients, respectively [14]. Lower baseline serum calcium levels and estimated glomerular filtration rate (eGFR) were major predictors for hypocalcemia [13, 14]. Hypocalcemia occurred approximately 7 to 20 days after the first dose of denosumab and reached a nadir of low calcium levels in the first 2 weeks up to 2 months [15]. Among patients who developed hypocalcemia, the median time to serum calcium nadir after denosumab administration was 3 weeks, in general approximately 1 - 2 weeks, and median time to correction of hypocalcemia was by 4 weeks [4, 5, 7, 12].
Denosumab inhibits osteoclastic bone resorption, leading to hypocalcemia by reducing calcium mobilization from the bone into the bloodstream, and denosumab is more potent than zoledronic acid [8, 13]. The risk factors for denosumab-associated hypocalcemia include bone metastatic cancer, renal impairment, vitamin D deficiency, the lack of prophylactic supplementation of calcium and/or vitamin D, preexisting hypoparathyroidism, hypomagnesemia, and high bone turnover state as assessed by turnover markers [2, 4, 7-9, 16-18]. Additionally, concomitant use of medications that increase calcium loss or potentiate the effect of denosumab (e.g., glucocorticoids, some anticonvulsants, bisphosphonates, and calcimimetics) increases the risk of hypocalcemia [4, 17].
Patients with chronic kidney disease (CKD) are at greater risk of developing hypocalcemia, likely due to CKD-associated secondary hyperparathyroidism, which places greater dependence on PTH-mediated bone resorption for maintenance of serum calcium [9]. Denosumab should be administered with caution in patients with CKD, because the drug lowers bone turnover rapidly and substantially and blocks calcium mobilization from bone in defense of hypocalcemia [2]. In a meta-analysis of six observational studies with 84 patients with end-stage renal disease (ESRD), the incidence of denosumab-associated hypocalcemia was as high as 42%. There were significant reductions in PTH and significant increases in BMD. The authors cautioned against the use of denosumab as the treatment of choice in ESRD patients although there were significant increases in BMD [15]. In a retrospective study performed in 14 patients with CKD stages 4 - 5 treated with denosumab, a 3.1-fold rise in the mean PTH level was observed at the time of the corrected calcium nadir [18]. Denosumab-induced hypocalcemia usually lasts for few weeks and the risk has been found to be highest in the first 2 weeks [18]. In a recent review, risk factors for hypocalcemia with denosumab use in CKD include lower baseline serum calcium and 25(OH)D and both low and high bone turnover [19]. Recent data have suggested a high bone turnover state as a risk factor for denosumab-associated hypocalcemia, because patients dependent on high bone turnover to maintain normal serum calcium concentrations are more sensitive to bone turnover inhibition by denosumab [6, 7].
The prevention of denosumab-associated hypocalcemia via close monitoring of the serum calcium, vitamin D, magnesium, phosphate, and kidney function is important. It is recommended in patients predisposed to hypocalcemia and disturbances of mineral balance within 14 days of denosumab injection [2, 9]. The importance of identifying patients at risk for hypocalcemia and addressing this risk by assuring an adequate intake of calcium and vitamin D before initiating therapy is also emphasized. Serum calcium levels may be checked prior to each dose of denosumab. Individuals at risk for hypocalcemia should be educated about the importance of calcium and vitamin D supplementation throughout long-term treatment, and about the signs and symptoms of hypocalcemia [2, 4].
This is a case of hypocalcemia after the administration of denosumab for the treatment of osteoporosis in a patient with coexisting but unrecognized severe vitamin D deficiency. Before administering denosumab, the patient’s calcium and vitamin D levels were not evaluated and calcium and vitamin D supplementation was not taken, both of which are incompatible with the current recommendation. However, serum calcium and 25(OH)D levels were later found to be low, with elevated serum PTH level and normal renal function and magnesium level, which indicated that hypocalcemia and secondary hyperparathyroidism were caused by severe vitamin D deficiency. Low urinary calcium also supported that the cause of hypocalcemia was due to vitamin D deficiency and secondary hyperparathyroidism. Inadequate exposure to sunlight, older age, and anti-tuberculosis medication were associated with lower vitamin D levels in this case. Additionally, secondary hyperparathyroidism could not increase 1,25(OH)2D adequately due to severe vitamin D deficiency, leading to aggravated hypocalcemia and hypophosphatemia in this patient. Secondary hyperparathyroidism maintains serum calcium in the normal range at the expense of mobilizing calcium from the skeleton and increasing calcium reabsorption and phosphorus wasting in the kidneys [20]. Although this patient had hypophosphatemia due to severe vitamin D deficiency, hyperphosphaturia by secondary hyperparathyroidism resulted in severe and prolonged hypophosphatemia. Hypophosphatemia has been shown to occur in 2.1% of patients administered with denosumab [20].
Learning points
Vitamin D deficiency is common but one of the modifiable risk factors for denosumab-associated hypocalcemia. Prior to denosumab initiation, it is recommended that pretreatment serum calcium and 25(OH)D levels are measured, and deficiencies are corrected. Serum calcium levels should be monitored during and after denosumab treatment, especially for 2 - 3 weeks.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Data Availability
The data supporting the finding of this study are available from the corresponding author upon reasonable request.
Abbreviations
25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; BMD: bone mineral density; CKD: chronic kidney disease; CT: computed tomography; ESRD: end-stage renal disease; eGFR: estimated glomerular filtration rate; IV: intravenous; MRI: magnetic resonance imaging; PTH: parathyroid hormone; RANK: receptor activator of nuclear factor kappa-B; RR: relative risk; SREs: skeletal-related events; TRP: tubular reabsorption of phosphorus
| References | ▴Top |
- Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwinski E, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513-523.
doi - Eastell R, Rosen CJ. Response to Letter to the Editor: "Pharmacological management of osteoporosis in postmenopausal women: an endocrine society clinical practice guideline". J Clin Endocrinol Metab. 2019;104(8):3537-3538.
doi pubmed - Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1-46.
doi pubmed - Pittman K, Antill YC, Goldrick A, Goh J, de Boer RH. Denosumab: Prevention and management of hypocalcemia, osteonecrosis of the jaw and atypical fractures. Asia Pac J Clin Oncol. 2017;13(4):266-276.
doi pubmed - Watthanasuntorn K, Abid H, Gnanajothy R. Severe hypocalcaemia following denosumab in a patient with cancer with vitamin D deficiency. BMJ Case Rep. 2018;11(1):e226727.
doi pubmed - Ishikawa K, Nagai T, Sakamoto K, Ohara K, Eguro T, Ito H, Toyoshima Y, et al. High bone turnover elevates the risk of denosumab-induced hypocalcemia in women with postmenopausal osteoporosis. Ther Clin Risk Manag. 2016;12:1831-1840.
doi pubmed - Ishikawa K, Nagai T, Tsuchiya K, Oshita Y, Kuroda T, Ito H, Tani S, et al. High bone turnover status as a risk factor in symptomatic hypocalcemia following denosumab treatment in a male patient with osteoporosis. Clin Interv Aging. 2018;13:1929-1934.
doi pubmed - Nasser SM, Sahal A, Hamad A, Elazzazy S. Effect of denosumab versus zoledronic acid on calcium levels in cancer patients with bone metastasis: A retrospective cohort study. J Oncol Pharm Pract. 2019;25(8):1846-1852.
doi pubmed - Laskowski LK, Goldfarb DS, Howland MA, Kavcsak K, Lugassy DM, Smith SW. A RANKL wrinkle: denosumab-induced hypocalcemia. J Med Toxicol. 2016;12(3):305-308.
doi pubmed - Qi WX, Lin F, He AN, Tang LN, Shen Z, Yao Y. Incidence and risk of denosumab-related hypocalcemia in cancer patients: a systematic review and pooled analysis of randomized controlled studies. Curr Med Res Opin. 2013;29(9):1067-1073.
doi pubmed - Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, Richardson GE, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082-3092.
doi pubmed - Body JJ, Bone HG, de Boer RH, Stopeck A, Van Poznak C, Damiao R, Fizazi K, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812-1821.
doi pubmed - Tsvetov G, Amitai O, Shochat T, Shimon I, Akirov A, Diker-Cohen T. Denosumab-induced hypocalcemia in patients with osteoporosis: can you know who will get low? Osteoporos Int. 2020;31(4):655-665.
doi pubmed - Kim KJ, Hong N, Lee S, Kim M, Rhee Y. A simple-to-use score for identifying individuals at high risk of denosumab-associated hypocalcemia in postmenopausal osteoporosis: a real-world cohort study. Calcif Tissue Int. 2020;107(6):567-575.
doi pubmed - Thongprayoon C, Acharya P, Acharya C, Chenbhanich J, Bathini T, Boonpheng B, Sharma K, et al. Hypocalcemia and bone mineral density changes following denosumab treatment in end-stage renal disease patients: a meta-analysis of observational studies. Osteoporos Int. 2018;29(8):1737-1745.
doi pubmed - Daga N, Joseph F. Denosumab-induced severe hypocalcaemia in a patient with vitamin D deficiency. BMJ Case Rep. 2020;13(8):e234508.
doi pubmed - Marlow CF, Sharma S, Babar F, Lin J. Severe hypocalcemia and hypomagnesemia with denosumab in advanced chronic kidney disease: case report and literature review. Case Rep Oncol Med. 2018;2018:2059364.
doi pubmed - Bhanot RD, Kaur J, Bhat Z. Severe hypocalcemia and dramatic increase in parathyroid hormone after denosumab in a dialysis patient: a case report and review of the literature. Case Rep Nephrol. 2019;2019:3027419.
doi pubmed - Gopaul A, Kanagalingam T, Thain J, Khan T, Cowan A, Sultan N, Clemens KK. Denosumab in chronic kidney disease: a narrative review of treatment efficacy and safety. Arch Osteoporos. 2021;16(1):116.
doi pubmed - Strickling J, Wilkowski MJ. Severe, Symptomatic hypocalcemia due to denosumab administration: treatment and clinical course. Case Rep Nephrol Dial. 2019;9(1):33-41.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
