| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 5, Number 4, August 2015, pages 250-255
Prevalence and Clinical Characteristics of Pretibial Myxedema in Chinese Outpatients With Thyroid Diseases
Changgui Lana, b, Wei Chena, Jing Zhaoa, Can Lia, Xiaofeng Meia, Jie Hua
aDepartment of Dermatology, China National Nuclear Corporation 416 Hospital, No. 4 Er Huan Lu Bei Si Duan, Chengdu 610051, Sichuan, China
bCorresponding Author: Changgui Lan, Department of Dermatology, China National Nuclear Corporation (CNNC) 416 Hospital, No. 4 Er Huan Lu Bei Si Duan, Chengdu 610051, Sichuan, China
Manuscript accepted for publication August 18, 2015
Short title: Prevalence and Clinical Manifestation of PTM
doi: http://dx.doi.org/10.14740/jem306e
| Abstract | ▴Top |
Background: There have been no reports on the prevalence of pretibial myxedema (PTM) in thyroid diseases in China. In order to resolve the problem, we retrospectively investigated Chinese outpatients with thyroid diseases and analyzed the clinical manifestation of PTM.
Methods: All outpatients with thyroid diseases at Department of Nuclear Medicine from October 24, 2000 to November 11, 2006 were included in the investigation by eligible case criteria and screened by PTM diagnosis criteria. The fill-out forms of PTM had three main items which contained demographics, diagnosis of thyroid diseases, relationship among I-131 therapy, thyroid function and onset of PTM, and the clinical manifestation of PTM. Descriptive statistics of the data were performed with statistical software SPSS17.0.
Results: The prevalence of PTM was 1.6% (728/44,646) in thyroid diseases, 1.7% in thyrotoxicosis, and 0.36% in Hashimoto thyroiditis, primary hypothyroidism and thyroid adenoma, respectively. The average age was 41.1 ± 11.9 years (15 - 78 years). The sex ratio was 1:3.7. Eighty-three percent of cases were Chinese farmers. The onset of PTM was 63.9% in euthyroidism, 22% in hyperthyroidism, 11.4% in hypothyroidism and 2.7% in unclear thyroid function. The course was 10 days to 10 years and its average was 37.8 ± 20.5 months. The clinical forms were 82.6% in non-pitting diffuse swelling, 12.4% in plaque/nodule, 2.7% in verruciform plaque, 1.6% in elephantiasis and 0.7% in tumorous.
Conclusions: The prevalence of PTM with chronic and autoimmune features is 1.6% in thyroid diseases. Its onset is not related to thyroid dysfunction and it should be treated early.
Keywords: Pretibial myxedema; Prevalence; Thyroid disease; Thyroid dysfunction; Clinical manifestation; I-131 treatment
| Introduction | ▴Top |
Pretibial myxedema (PTM) or localized myxedema or thyroid dermopathy had often been thought to be an extrathyroidal manifestation of thyrotoxicosis (Graves’ disease), since Hektoen reported the first case of PTM associated with hyperthyroidism in 1895 [1]. It was often seen by endocrine physicians at hyperthyroidism treatment centers but rarely met by dermatologists at general dermatology department. The prevalence of PTM in patients with thyrotoxicosis was reported to be 0.6-4.3% during the 1960s in west countries [2-4]. However, in 1.3 billion population country, China, there were few dermatologists who specialized in PTM research. The diagnosis and treatment of PTM had been a problem at department of dermatology. More unfortunately, little information was available regarding the prevalence of PTM in patients with thyroid diseases in China. In order to resolve the problem, we retrospectively investigated outpatients with thyroid diseases at Department of Nuclear Medicine of CNNC 416 Hospital and analyzed the clinical manifestation of PTM.
| Materials and Methods | ▴Top |
All outpatient cases with thyroid diseases in the archive of Department of Nuclear Medicine in CNNC 416 Hospital from October 24, 2000 to November 11, 2006 were included in the investigation. The cases were excluded which lacked history records, or physical examination (including skin), or thyroid function test, or thyroid disease diagnosis. The diagnosis criteria of PTM included: 1) the cases were suffering from thyroid diseases or had thyroid disease history; 2) the cases had non-pitting swelling of skin or nodule or plaque or elephantiasis; 3) skin lesions were excluded from erysipelas, nodular vasculitis, keloid and other mucinoses such as cutaneous focal mucinosis, lichen myxedematosus, etc.
All eligible outpatient cases were screened on the basis of the PTM diagnosis criteria. The cases which were qualified for the PTM diagnosis criteria were recorded into the preliminary forms which had been designed and printed before screening. The form had three main items which must be filled out. The first item was the demographic data field. It contained the case number, outpatient’s name, sex, age, occupation, address, and telephone number. The second item was the diagnosis of thyroid diseases and its treatment with I-131 including names of thyroid diseases, thyroid function test (normal, hyperthyroidism and hypothyroidism decided by T3, T4, FT3, FT4 and TSH), whether receiving I-131 treatment or not. The third item was the clinical manifestation of PTM, in which the onset of PTM was before, meanwhile or after thyroid dysfunction; thyroid function when PTM occurred; local injuries, course of PTM and lesional types (non-pitting swelling, nodule, plaque, and elephantiasis) were also included.
After the forms were collected, PTM cases were reconfirmed by telephone contact or they were asked to come back and re-examined by dermatologists if could.
Finally, the data were analyzed with statistical software SPSS17.0. Variables were analyzed by descriptive statistics such as frequencies, percentages, means, standard derivation and range.
| Results | ▴Top |
Prevalence of PTM in Chinese outpatients with thyroid diseases
In 6 years from October 24, 2000 to November 11, 2006, 44,646 cases with thyroid diseases were eligible from outpatient archives of Department of Nuclear Medicine. Among them, 42,417 cases were in thyrotoxicosis (Graves’ disease), 1,393 cases in Hashimoto thyroiditis, 557 cases in primary hypothyroidism and 279 cases in thyroid adenoma. However, 728 cases with PTM were diagnosed totally. Seven hundred twenty cases were in thyrotoxicosis, five cases in Hashimoto thyroiditis, two cases in primary hypothyroidism and only one case in thyroid adenoma. Therefore, the prevalence of PTM was 1.6% (728/44,646) in thyroid diseases. Further analyzing, the prevalence of PTM was 1.7% (720/42,417) in thyrotoxicosis, 0.36% (5/1,393) in Hashimoto thyroiditis, 0.36% (2/557) in primary hypothyroidism and 0.36% (1/279) in thyroid adenoma. About 98.9% (720/728) of PTM were associated with thyrotoxicosis or thyrotoxicosis history and 1.1% with other thyroid diseases.
Demographic characteristics of 728 PTM cases
Among 728 PTM cases, 154 cases were male and 574 cases were female. The ratio of male to female was 1:3.7. The age ranged from 15 to 78 years old and the average of the age was 41.1 ± 11.9 years. Occupation of PTM patients included Chinese farmer, city resident, worker, teacher, cadre, doctor and police (Table 1).
 Click to view | Table 1. Demographic Characteristics of 728 PTM Cases |
The ratio of sex varied with the change of decades in life. In the second decade of life, the ratio of male to female was 1:3.5, but to the third decade of life, it went up to the peak and female cases were 7.2 times of male cases (1:7.2). Then the ratio went down gradually to the lowest value of about 1:1 in the eighth decade of life.
The age distribution of 728 PTM cases in the decades of life is shown in Figure 1. The highest peak was in the fourth decade and the two ends in decades of life had the least cases. The percentages of PTM cases were 3.71%, 14.70%, 34.20%, 23.76%, 17.72%, 4.95% and 0.96% in the second, third, fourth, fifth, sixth, seventh, and eighth decades of life respectively. Therefore, the majority (90.4%) were located in the third, fourth, fifth and sixth decades. The data demonstrated the major were main labors in their families.
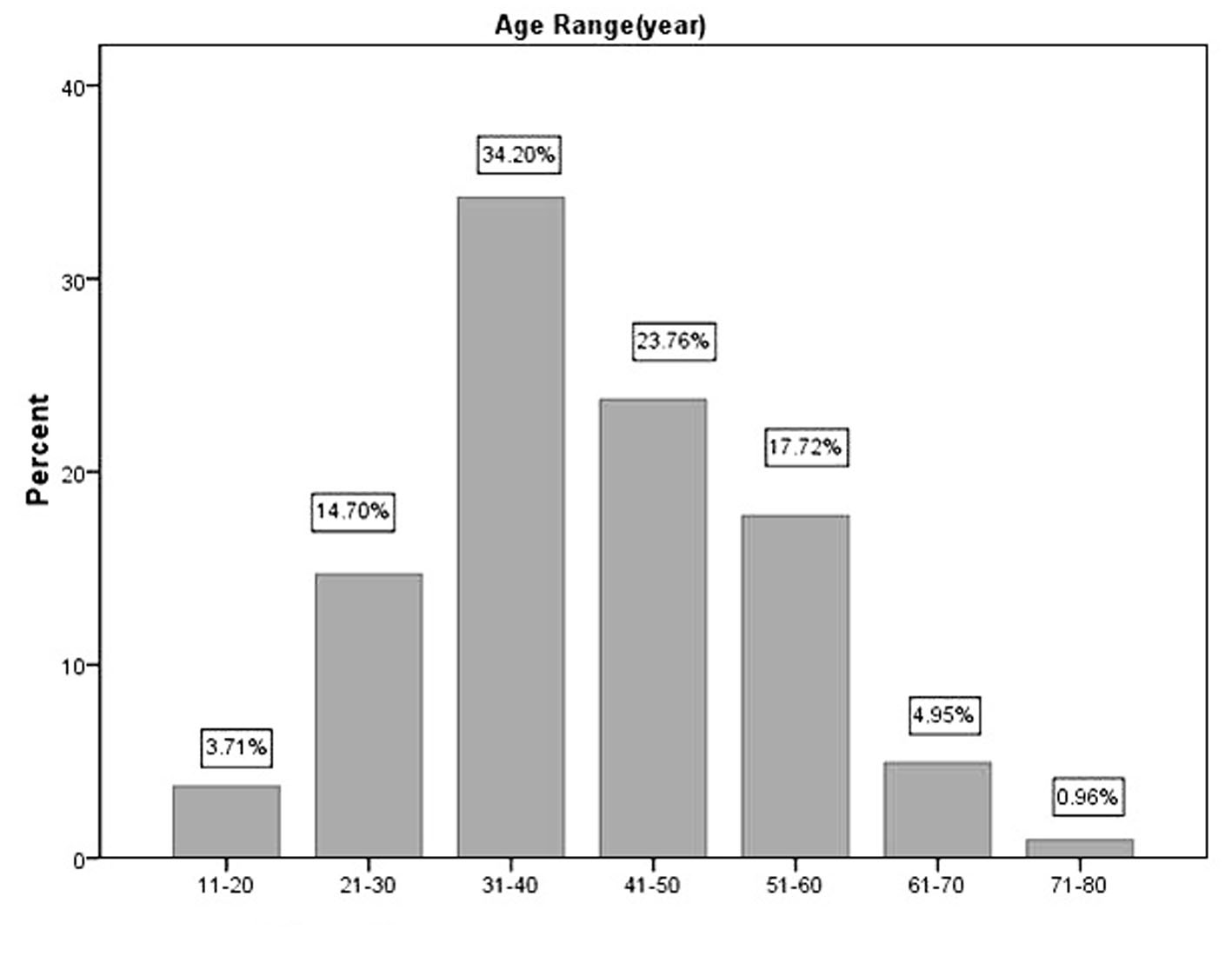 Click for large image | Figure 1. Age distribution of 728 PTM cases in decades of life. |
The 728 PTM cases consisted of 604 Chinese farmers, 81 city residents, 17 workers, 11 teachers, 10 cadres, three doctors and two police. The percentages of them in the occupation were 83%, 11.1%, 2.3%, 1.5%, 1.4%, 0.4% and 0.3% in Chinese farmers, city residents, workers, teachers, cadres, doctors and police respectively. In all decades of life, Chinese farmers were the majors. The data indicated most (83%) were Chinese farmers.
Clinical manifestation of 728 PTM cases
The courses of 728 PTM cases ranged from 10 days to 10 years. The average of their courses was 37.8 ± 20.5 months. The lesions of 728 PTM cases occurred on anatomic sites of the body as follows: 660 cases (90.7%) on the bilateral pretibial regions, 30 cases (4.1%) on the bilateral lower legs, 14 cases (1.9%) on the bilateral lower extremities, four cases on the bilateral pretibial regions and toes, six cases on the bilateral lower legs and toes, three cases on the bilateral lower legs and feet, two cases on the whole legs and feet below bilateral knees, one case on the bilateral lower legs and ankles, one case on the bilateral pretibial regions and interscapular region, one case on the bilateral pretibial regions and left index finger, one case on the bilateral extensors of lower legs and left shoulder, two cases on the right pretibial regions and three cases on the left pretibial regions. Totally, the involvements of both sides were 723 cases and occupied 99.3%. Only five cases were of unilateral distribution and was 0.7%. Ten cases were involved in toes, one case in the finger, one case in the shoulder and one case in the interscapular. In our 728 cases, half of them had definite local injury history or scars on lesions. The ones of another half were unclear.
The onset of PTM often occurred at bilateral extensors of lower legs and presented itself as erythema or small nodule or local erythematous, non-pitting swelling. The lesions, gradually and insidiously, developed into indurated nodules, or local erythematous plaques, or non-pitting diffuse swelling without temperature and pain. Some of lesions had local hyperhidrosis and/or terminal hairs. Severe cases manifested as elephantiasis, or tumorous, or giant plaque. In our 728 PTM cases, 601 cases were erythematous, non-pitting diffuse swelling at lower legs. Ninety cases were indurated nodules and plaques (plaque was major and nodule was minor as plaque/nodule, vice versa as nodule/plaque). Twenty cases were verruciform plaques. Twelve cases and five cases were elephantiasis and tumorous respectively. The percentage of different PTM types is shown in Figure 2. Non-pitting diffuse swelling at lower legs was most and occupied 82.55% in the 728 cases.
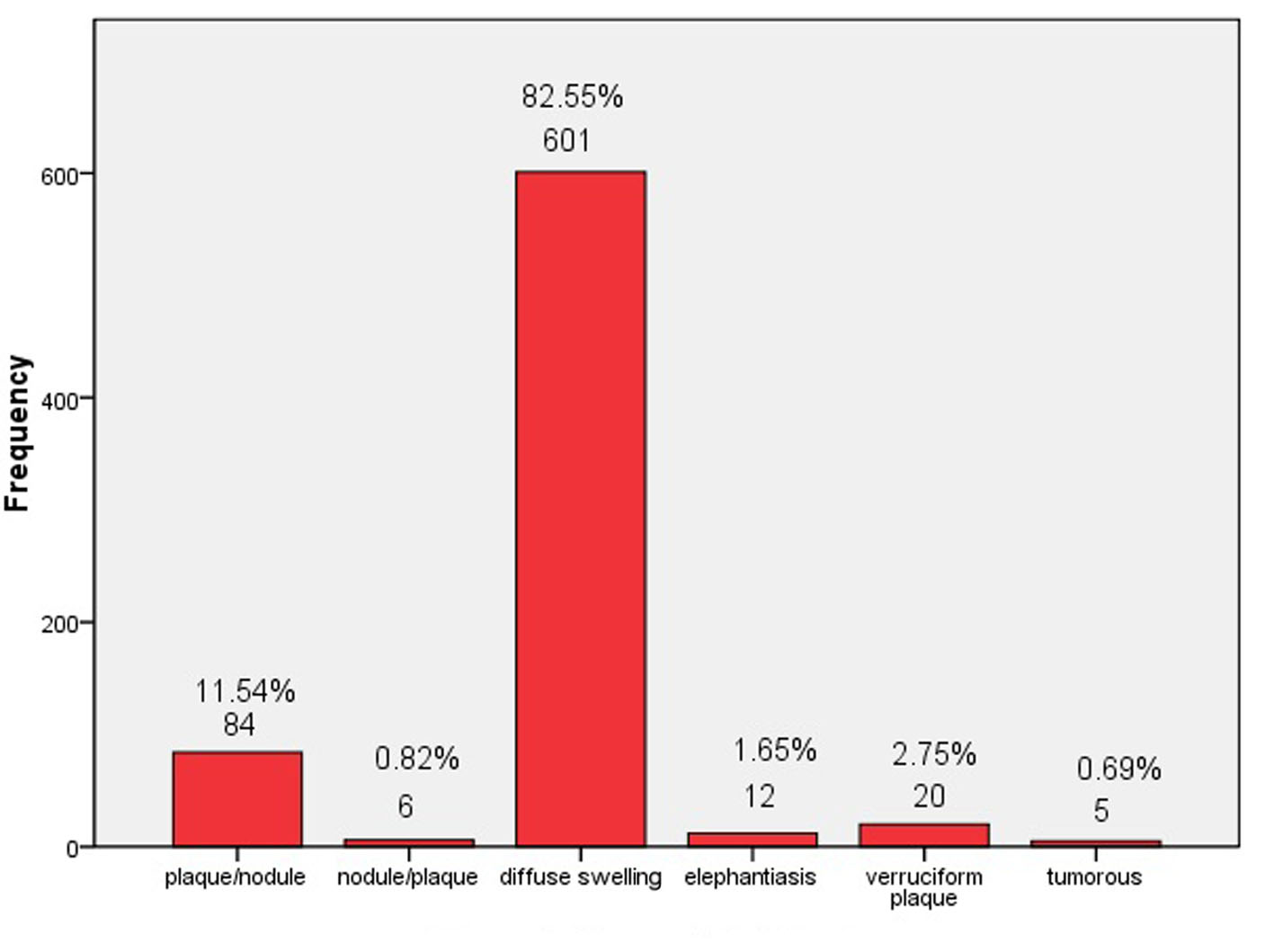 Click for large image | Figure 2. Types of PTM lesions. |
Relationship among PTM, thyroid function and I-131 treatment
In 728 PTM cases, 103 cases did not receive I-131 treatment but got medicine or surgery or no treatment. When PTM occurred, 68 cases were hyperthyroidism, 26 cases were hypothyroidism and nine cases were euthyroidism. On the contrary, 625 cases got I-131 treatment. Among them, 70 cases occurred before getting I-131 and 555 cases occurred after getting I-131. In the 625 cases, 92 cases were hyperthyroidism, 57 cases were hypothyroidism and 456 cases were euthyroidism when PTM occurred. In about half of the euthyroid cases after I-131 treatment, hypothyroidism was corrected by supplement of thyroxine. It was in the state of recovered thyroid function that PTM occurred. In addition, there were 20 cases whose thyroid functions were unclear (Table 2). And more interesting, in 728 PTM cases, two cases happened before occurrence of hyperthyroidism of Graves’ disease. These data demonstrated that these cases, whether they got I-131 treatment or not, would suffer from PTM. Also, no matter what their thyroid functions were, high or low or normal, PTM would happen as usual. Therefore, the onset of PTM might have no relationship with I-131 treatment and thyroid function.
 Click to view | Table 2. Relationship Among PTM, I-131 Treatment and Thyroid Function |
| Discussion | ▴Top |
There have been few reports on the prevalence of PTM in thyrotoxic patients and thyroid diseases in China. In the investigation, we have found that the prevalence of PTM is 1.7% in Chinese thyrotoxicosis, 0.36% respectively in Hashimoto thyroiditis, primary hypothyroidism and thyroid adenoma, and totally 1.6% in thyroid diseases. However, the prevalence of PTM in thyrotoxicosis was reported to be 4.3% in London and 1% in America, but in Singapore it was only 0.6%. Recently it is estimated by Fatourechi that the prevalence of PTM in Graves’ patients is approximately 1.5 % [5]. The estimated value is close to our finding. Since the prevalence of thyrotoxicosis is 0.4% [6], the distribution of Graves’ disease around the globe, so far as data are available, appears to be relatively equal, affecting all countries and races [7]. In the 1.3 billion population, China has 6.5 million thyrotoxic patients and 110.5 thousand PTM patients. Therefore, PTM is not rare in China and is worthy of being research.
The first case of PTM was reported to be associated with Graves’ disease. Gradually, it was found to occur also in Hashimoto’s thyroiditis [8], primary hypothyroidism [9] and in thyroid adenoma [10], even in thyroid cancer [11]. The data fully demonstrate that the background of thyroid dysfunction may play a role in the pathogenesis of PTM because the above-mentioned associated thyroid diseases of PTM belong to a group of autoimmune thyroid diseases (AITD) and they have autoimmunity in common. By the way, our investigated results did not indicate PTM occurred in thyroid cancer just because our Department of Nuclear Medicine predominately used I-131 to treat hyperthyroidism not thyroid cancer.
In the investigation, sex ratio of PTM is 1:3.7 of male to female. Female cases are dramatically more than male cases. When PTM patients are in the third decade of life and their activities of sex glands are the highest, the ratio reaches the highest peak, that is, 1:7.2 of male to female. When they are in the eighth decade of life and their activities of sex glands are the lowest, the ratio closes to 1:1 and there is no sex difference in the PTM cases. Generally considered, it is characteristic of autoimmune diseases that the sex ratio varies with the change of ages and activities of sex glands. So sex ratio of PTM in decades of life is the second characteristic with autoimmunity.
A lot of PTM cases with hyperthyroidism have been reported since the first case was reported. Recently, PTM has been reported to occur in hypothyroid patients [9, 12]. In a series of 178 PTM cases [13], 91.0% (162) cases were hyperthyroid and 3.9% (seven) were hypothyroid. In our investigation, 22% (160) cases occurred in the hyperthyroidism and 11% (83) cases occurred in the hypothyroidism. The association between PTM and thyroid dysfunction has chronically allowed people to believe that PTM might be caused by thyroid dysfunction and it would gradually subside after correcting the dysfunction. In fact, the onset of PTM may occur before the hyperthyroidism of Graves’ disease or after correcting the hyperthyroidism of Graves’ diseases. Even if PTM simultaneously appeared in thyroid dysfunction such as hyperthyroidism or hypothyroidism, PTM would not relieve after correcting the thyroid dysfunction in our investigation. However, the onset of PTM in euthyroid patients has been reported in six cases [14-16]. In our investigation, two PTM cases occurred in the euthyroid state before the appearance of hyperthyroidism of Graves’ disease. What is more, 64% (465) cases occurred at the euthyroid state after hyperthyroidism was treated by I-131 or antithyroid drugs or surgery. Since PTM occurs under any thyroid function states and it will not relieve after correcting the thyroid dysfunction, it is not thyroid dysfunction to cause PTM. It is more likely the autoimmune background of thyroid dysfunction plays an important role in the pathogenesis of PTM. More and more evidences keep accumulating in the autoimmune pathogenesis of PTM. Antigen-specific T lymphocytes infiltrating the pretibial lesions are of primary immune response [17], high serum levels of TRAbs are found in all PTM patients [18], TSH receptors exist on the dermal fibroblasts of pretibial skin [19], and IgG, IgA and C3 deposit in the pretibial lesions of PTM [20]. Therefore, PTM is considered to be a local autoimmune entity associated with autoimmune thyroid diseases [21, 22], in which cell- mediated and humoral immunity (TRAb) plays a role in the pathogenesis [23].
The course of PTM is 10 days to 10 years and its average is 37.8 months (more than 3 years). The natural course and long-term outcome of PTM have been reported in a series of 178 patients that 50% of mild cases had no improvement within 17 years and all five cases of elephantasis had no remission after 25 years of follow-up [13]. Our experience is that PTM is rarely transient but most is persistent for years, even more than 10 years (unpublished randomized controlled trial). So PTM is a chronic dermopathy.
Involvement of anatomic sites predominantly distributes on the bilateral lower legs (99.3%), whereas the unilateral lower leg is rarely involved (0.7%). Except for lower legs, other sites are also involved such as the foot, hand, toe and finger, shoulder and back. Although the etiology of PTM is unclear, local injury may be a precipitating factor because half of PTM cases have definite injury history or obvious scars on lesions. As for lesional types of 728 PTM cases, non-pitting diffuse swelling occupies 82.6% and lies in the first. Next is plaques or nodules (12.4%), severe is the least (5%), such as elephantiasic, verruciform plaque and tumorous types. While Schwartz et al [13] reported the non-pitting diffuse edema (43.3%) was the most prevalent form of PTM. The other clinical forms were respectively plaque in 27.0%, nodular in 18.5%, elephantiasic in 2.8% and unclassifiable and/or unknown in 8.4%. However severe PTM obviously interferes with normal lives and works of patients. In our investigation, most of PTM patients are Chinese farmer and in the third, fourth, fifth and sixth decades of life. They are main labors in their families. So, once PTM occurs, it has to be treated early [24] in order to prevent it from developing into severe types and then influencing family labor supply.
Acknowledgement
We thank Bin Yan, MD, Vice-Director, Yanwu Dong, MD, Vice-Director and Qi Chen, MD, Director at Department of Nuclear Medicine and all staffs at Department of Dermatology in CNNC 416 Hospital for their supports to this work.
Disclosure Statement
The authors have nothing to disclose.
| References | ▴Top |
- Watson EM. Localized (pretibial) myxoedema associated with Graves' disease. Can Med Assoc J. 1946;54:260-265.
- Gimlette TM. Pretibial myxoedema. Br Med J. 1960;2(5195):348-351.
doi pubmed - Shasky D, Nelson J. Pretibial myxedema. Arch Dermatol. 1966;94(5):658-660.
doi pubmed - Kee CW, Cheah JS. Thyrotoxic pretibial myxoedema in Asian patients in Singapore. Postgrad Med J. 1975;51(596):407-409.
doi - Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves' disease: a 2014 update. J Endocrinol Invest. 2014;37(8):691-700.
doi pubmed - McLeod DS, Cooper DS, Ladenson PW, Whiteman DC, Jordan SJ. Race/Ethnicity and the prevalence of thyrotoxicosis in young Americans. Thyroid. 2015;25(6):621-628.
doi pubmed - DeGroot LJ: Graves' Disease and the Manifestations of Thyrotoxicosis. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, et al., eds. Endotext. South Dartmouth (MA), 2000.
- Stewart G, Payne CM, Croft DN. Hashimoto's thyroiditis associated with dysthyroid eye disease, pretibial myxoedema and thyroid acropachy. J R Soc Med. 1984;77(3):240-243.
pubmed - Kriss JP. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16(2):409-415.
pubmed - Marconi B, Brandozzi G, Galeazzi A, Campanati A, Simonetti O, Santinelli A, Pisa E, et al. A case of pretibial myxoedema associated to ectopic secreting thyroid nodule on thyroglossal duct residue. J Eur Acad Dermatol Venereol. 2008;22(5):620-621.
doi pubmed - Bosadjieva E, Georgieva S, Altunkov P, Popov K, Koeva L, Genowa M. Localized pretibial myxedema and thyroid acropachy in a case of Hurthle cell adenocarcinoma. Int J Dermatol. 1971;10(3):170-174.
doi pubmed - Dharmalingam M, Seema G, Khaitan B, Karak A, Ammini AC. Plaque form of pretibial myxedema in hypothyroidism. Indian J Dermatol Venereol Leprol. 2001;67(6):330-331.
pubmed - Schwartz KM, Fatourechi V, Ahmed DD, Pond GR. Dermopathy of Graves' disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87(2):438-446.
pubmed - Senel E, Gulec AT. Euthyroid pretibial myxedema and EMO syndrome. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18(1):21-23.
- Nair PA, Mishra A, Chaudhary A. Pretibial Myxedema Associated with Euthyroid Hashimoto's Thyroiditis: A Case Report. J Clin Diagn Res. 2014;8(6):YD01-02.
doi - Verma S, Rongioletti F, Braun-Falco M, Ruzicka T. Preradial myxedema in a euthyroid male: A distinct rarity. Dermatol Online J. 2013;19(4):9.
pubmed - Heufelder AE, Bahn RS, Scriba PC. Analysis of T-cell antigen receptor variable region gene usage in patients with thyroid-related pretibial dermopathy. J Invest Dermatol. 1995;105(3):372-378.
doi pubmed - Vannucchi G, Campi I, Covelli D, Forzenigo L, Beck-Peccoz P, Salvi M. Treatment of pretibial myxedema with dexamethazone injected subcutaneously by mesotherapy needles. Thyroid. 2013;23(5):626-632.
doi pubmed - Daumerie C, Ludgate M, Costagliola S, Many MC. Evidence for thyrotropin receptor immunoreactivity in pretibial connective tissue from patients with thyroid-associated dermopathy. Eur J Endocrinol. 2002;146(1):35-38.
doi pubmed - Antonelli A, Palla R, Casarosa L, Fallahi P, Baschieri L. IgG, IgA and C3 deposits in the extra-thyroidal manifestations of autoimmune Graves' disease: their in vitro solubilization by intravenous immunoglobulin. Clin Exp Rheumatol. 1996;14(Suppl 15):S31-35.
pubmed - Lan C, Zhou G. Advances in the mechanisms of initiation and progression of pretibial myxedema. Int J Dermatol Venereol. 2010;36:155-158.
- Lan C, Li C, Yang M, Mei X, He Z, Chen W, Chen H, et al. Pretibial myxoedema with autoimmunity and hyperplasia treated with glucocorticoids and surgery. Br J Dermatol. 2012;166(2):457-459.
doi pubmed - Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6(5):295-309.
doi pubmed - Takasu N, Higa H, Kinjou Y. Treatment of pretibial myxedema (PTM) with topical steroid ointment application with sealing cover (steroid occlusive dressing technique: steroid ODT) in Graves' patients. Intern Med. 2010;49(7):665-669.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
